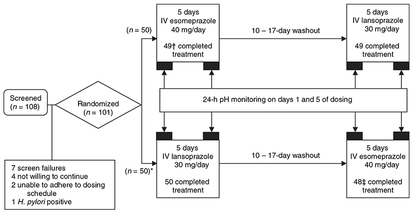
Figure 1.
Study design and subject disposition. *One subject was assigned to receive intravenous (IV) lansoprazole 30 mg followed by IV esomeprazole 40 mg, but received IV lansoprazole 30 mg in both treatment periods. The subject was discontinued after two doses of IV lansoprazole 30 mg were administered in the second treatment period. † One subject was discontinued from the intention-to-treat population due to study drug non-compliance, insufficient washout and lack of evaluable pH data. ‡ Two subjects were discontinued from the intention-to-treat population due to study drug non-compliance, insufficient washout and lack of evaluable pH data.