Table 3.
Matched pair comparison of structures similar to erlotinib and lapatinib (13-28).
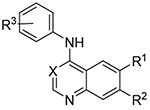 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | X | R3 | Mtb signala,b | WS-1c | ||
| 5 μM | 10 μM | 20 μM | (μM) | |||||
| 13 | CF3 | H | CH | 3,4,5-(OMe)3 | 1.23 | 1.14 | 1.21 | >100 |
| 14 | CF3 | H | N | 3,4,5-(OMe)3 | 1.25 | 1.12 | 1.06 | >100 |
| 15 | Br | H | CH | 3,4,5-(OMe)3 | 1.35 | 1.41 | 1.28 | >100 |
| 16 | OMe | OMe | CH | 3,4,5-(OMe)3 | 1.26 | 1.11 | 1.06 | >100 |
| 17 | OMe | OMe | N | 3,4,5-(OMe)3 | 1.03 | 1.01 | 0.94 | >100 |
| 18 | OMe | OMe | CN | 3,4,5-(OMe)3 | 1.03 | 1.01 | 0.97 | >100 |
| 19 | 6,7-(OCH2CH2OMe)2 | N | 3,4,5-(OMe)3 | 1.02 | 0.96 | 0.87 | >100 | |
| 20 | 6,7-(OCH2CH2OMe)2 | CH | 3-Ethynyl | 0.88 | 0.79 | 0.58 | >100 | |
| 21 | OMe | OMe | CH | 3-Ethynyl | 1.02 | 0.89 | 0.67 | >100 |
| 22 | OMe | OMe | N | 3-Ethynyl | 0.93 | 0.83 | 0.69 | >100 |
| 23 | OMe | OMe | CN | 3-Ethynyl | 1.08 | 1.03 | 0.98 | >100 |
| 24 | OMe | OMe | CH | 3-Bromo | 1.03 | 0.90 | 0.6 | 9.6 |
| 25 | OMe | OMe | N | 3-Bromo | 0.98 | 0.71 | 0.65 | 3.9 |
| 26 | OMe | OMe | CN | 3-Bromo | 0.88 | 0.56 | 0.37 | 11 |
| 27 | OMe | OMe | N | 3-Cl-4-(2-F-PhO) | 0.92 | 0.74 | 0.80 | 1.1 |
| 28 | OMe | OMe | CH | 3-Cl-4-(2-F-PhO) | 0.78 | 0.22 | 0.10 | 11 |
Relative luminescence was measured at 3 days after treatment. Values = RLU(sample)/RLU(no compound);
None of the compounds reduced the relative Mtb signal below 95 % at 1.3 or 2.5 μM;
IC50 (mean average n = 4), 48 h
