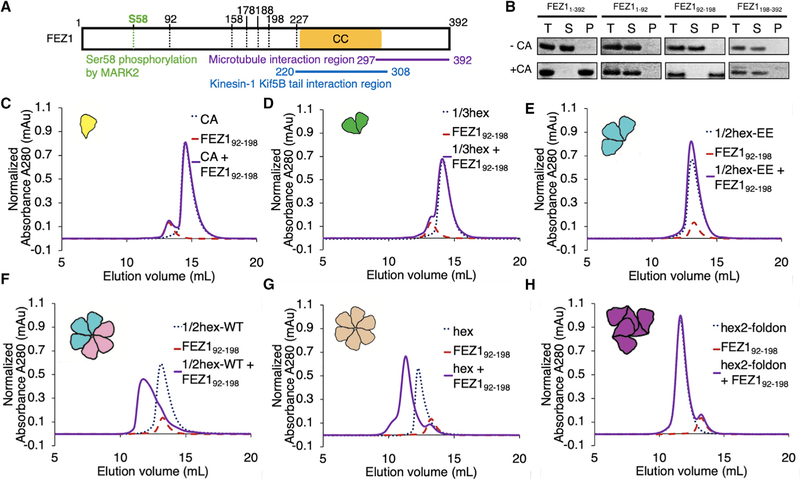
Figure 1. FEZ1 Is a Specific CA Hexamer Sensor.
(A) Schematic diagram of the FEZ1 constructs (marked by the black dotted lines) used for capsid-binding assays. The yellow bar indicates the predicted coiled-coil region of FEZ1. The putative functional regions of FEZ1 are labeled with different colors.
(B) Copelleting assay of FEZ11–392, FEZ11–92, FEZ192–198, and FEZ1198–392 with 14C/45C crosslinked CA tubes in 150 mM NaCl. Full-length FEZ11–392 and FEZ192–198 copellet with CA tubes, while the N-terminal (FEZ11–92) and C-terminal (FEZ1198–392) regions do not. T, total load; S, supernatant; P, pellet.
(C–H) Size-exclusion chromatography (SEC)-binding assay of FEZ192–198 with different CA assemblies (schematics shown in cartoon insets). The concentrations of FEZ1 and CA monomer in each binding reaction were 33 and 98 μM, respectively. FEZ192–198 does not coelute with CA monomer (C), 1/3-hexamer (D), or 1/2-hexamerEE (E) in SEC assays. There is no elution shift of the mixture (solid purple) relative to the individual components (dotted lines). FEZ192–198 does coelute with the CA hexamer (G), and restoration of a hexamer from two 1/2-hexamers (cyan and pink cartoons) restores FEZ192–198 coelution (F), which coelutes at the same position as with CA hexamers. In contrast, FEZ192–198 does not coelute with hexamer-2foldon (purple cartoon), which also contains 6 CA subunits but arranged around the 3-fold capsid interface (H).
See also Figure S2.