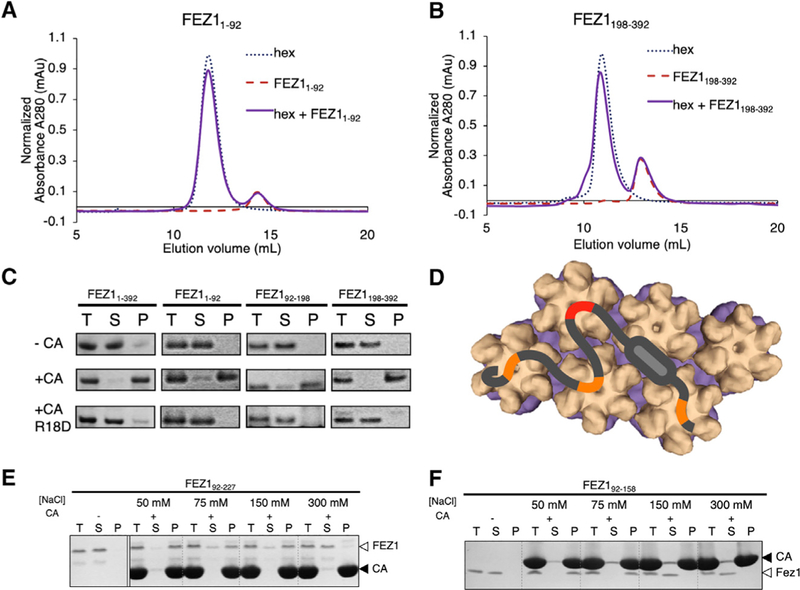
Figure 4. FEZ1 Has Multiple Negatively Charged Regions for Avid Binding to the Capsid.
(A andB) SEC-binding assay showing that FEZ11–92 (A) or FEZ1189–392 (B) does not coelute with CA hexamer. There is no elution shift of the mixture (solid purple) relative to the individual components (dotted lines).
(C) Copelleting assays show that capsid-binding sites in both FEZ11–92 and FEZ1189–392 can be detected in low salt (50 mM NaCl); the binding is abolished when using R18D CA tubes (bottom row).
(D) Cartoon schematic showing the interaction of Fez1 with multiple hexamers.
(E and F) Binding of FEZ1 constructs to CA tubes is reduced and abolished at different salt concentrations. FEZ192–227 interacts with CA tubes at physiological salt concentrations (150 mM) (E), while FEZ192–158 only binds at lower salt concentrations (F), consistent with multiple binding sites in FEZ1.