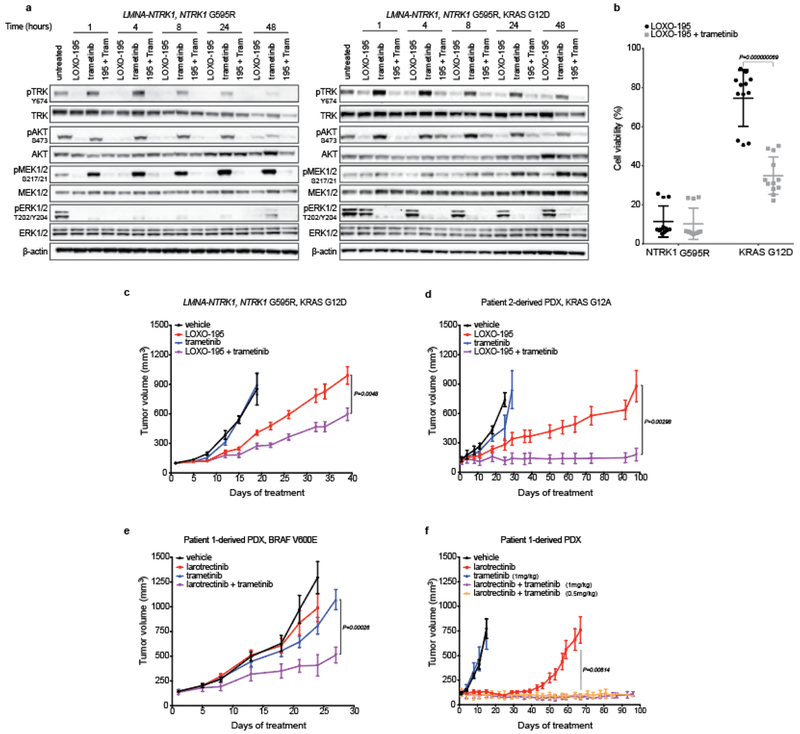
Fig. 3: Dual TRK and MEK blockade is required to inhibit tumor growth in TRK fusion-positive models that acquired MAPK alterations.
a, Western blots from the two colorectal cancer cell lines LMNA-NTRK1, NTRK1 G595R and LMNA-NTRK1, NTRK1 G595R, KRAS G12D, treated as indicated. LOXO-195 (50nM), trametinib (10nM) or the combination of both drugs (195 + tram) were administered at the indicated time and protein lysates were probed with the indicated antibodies. While LOXO-195 was sufficient to inhibit both phospo-TRK and phospho-ERK in the KRAS wild type cell line, the combination of LOXO-195 and trametinib was required for this dual inhibition in the KRAS G12D mutated cell line. Three biological replicates were performed. b, Proliferation assays on the same cell lines (labeled NTRK1 G595R and KRAS G12D, respectively) treated for 72 hours with LOXO-195 (125nM), trametinib (2nM) or their combination. Data are presented as mean ± SD of four biological replicates. Two-tailed unpaired t-test was used to evaluate significant differences in % of viable cells. P values <0.05 were considered statistically significant. c, In vivo efficacy of LOXO-195 (100mg/Kg BID 5 days a week), trametinib (1mg/kg 4 days a week) or their combination on xenofgrafts established from the LMNA-NTRK1, NTRK1 G595R, KRAS G12D cell line (vehicle n=5, LOXO-195 n=6, trametinib n=5, LOXO-195 + trametinib n=6). d, In vivo efficacy of LOXO-195 (100mg/Kg BID 5 days a week), trametinib (3mg/kg 4 days a week) or their combination on PDXs established from the KRAS G12A positive liver biopsy collected at the time of LOXO-195 progression from patient 2 (Figure 1d; vehicle n=4, LOXO-195 n=5, trametinib n=5, LOXO-195 + trametinib n=5). e, In vivo efficacy of larotrectinib (200mg/kg daily 5 days a week), trametinib (1mg/kg daily 4 days per week) and the combination of both drugs in larotrectinib-resistant PDXs established from Patient 1 (Patient 1-derived PDX, BRAF V600E, Figure 1a; vehicle n=8, larotrectinib n=8, trametinib n=8, larotrectinib + trametinib n=7). f, In vivo efficacy of larotrectinib (200mg/kg daily 5 days a week), trametinib (1mg/kg 4 days a week) and the combination of both drugs in larotrectinib-sensitive PDXs established from Patient 1 (Patient 1-derived PDX, Figure 1a; note that trametinib was also tested in combination with larotrectinib at half of the dose: 0.5mg/kg 4 days a week, orange line; vehicle n=6, larotrectinib n=8, trametinib n=7, larotrectinib + trametinib (1mg/kg) n=7, larotrectinib + trametinib (0.5mg/kg) n=7). Combination therapy prevents the development of primary or acquired resistance (ongoing at 3 months). Two-tailed unpaired t-test was used to evaluate significant differences in the tumor volumes. Data are presented as mean±SEM. P values <0.05 were considered statistically significant.