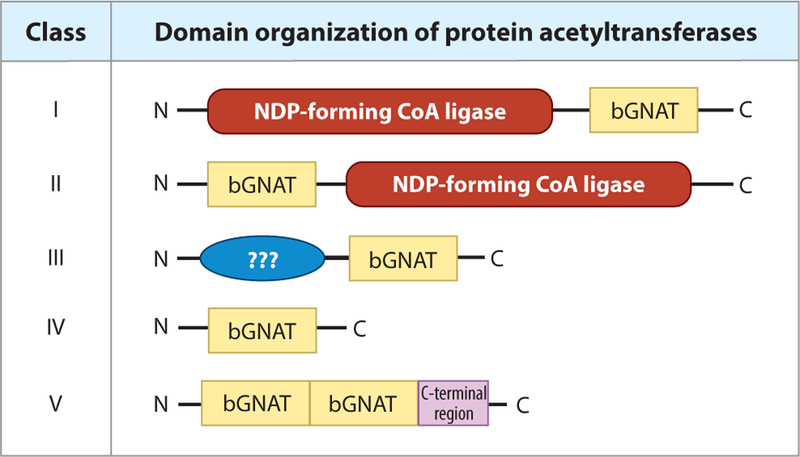
Figure 2.
Domain organization of bacterial protein acetyltransferases. Acetyltransferases belonging to classes I–IV have been identified within prokaryotic genomes. Type I acetyltransferases contain a large regulatory N-terminal domain of unknown function. These domains are homologous to NDP-forming CoA ligases, although they lack a critical active-site histidine residue and do not possess this activity. Within the C terminus of these proteins, a bGNAT catalytic domain carries out the acetylation event. Type II bGNATs are similar to type I, but their domain organization is reversed. The catalytic GNAT domain is within the N terminus, with a large regulatory domain on the C terminus. Type III bGNATs are also similar to type I, in that their regulatory domain lies on the N terminus. However, the regulatory domains of type III acetyltransferases are different in size from that of type I (~300–400 residues versus 800–900 residues, respectively). Type IV bGNATs are unique in that they only contain a catalytic bGNAT domain. It is thought that type I–III acetyltransferases target proteins while type IV are able to target both proteins and small molecules. Lastly, type V contains two sequential bGNAT domains before the C-terminal region. Abbreviations: bGNAT, bacterial GNAT; GNAT, Gcn5 histone N-acetyltransferase; NDP, nucleoside diphosphate.