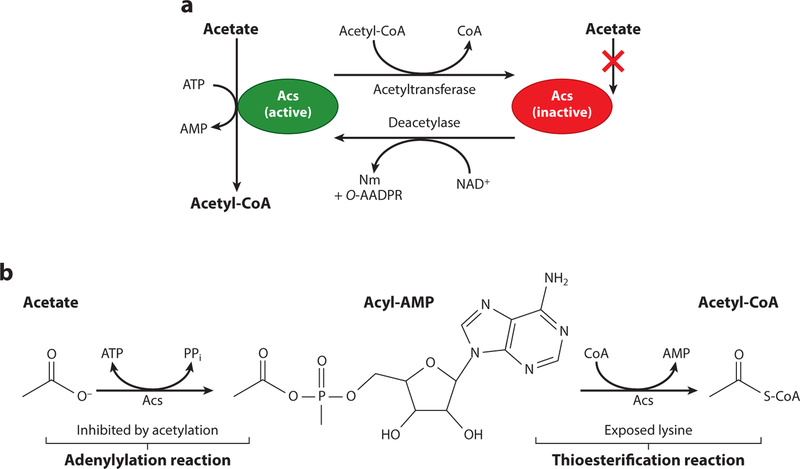
Figure 3.
Reversible lysine acetylation of Acs. (a) Pictured is a model of Acs reversible lysine acetylation as studied in Salmonella enterica. Acs is a central metabolic enzyme that under low-acetate concentrations (10 mM) activates acetate to acetyl-CoA (green protein) via an acetyladenylate intermediate with the release of pyrophosphate. A catalytic lysyl residue (K609) coordinates the carboxylic acid of acetate into the active site, and acetylation of K609 halts Acs activity (red protein). The acetyl moiety on K609 can be removed by a protein deacetylase, returning the enzyme to its active form. In S. enterica, the type I bGNAT, Pat, acetylates Acs and the NAD+-dependent sirtuin deacetylase, CobB, removes this modification. (b) Activation of acetate to acetyl-CoA occurs in a two-step reaction mechanism. In the adenylation reaction, K609 of Acs binds ATP and acetate, forming an acyl-AMP intermediate with the release of pyrophosphate. In an esterification reaction, AMP is replaced by CoA, releasing free AMP. It is in this conformation that the active-site lysyl residue is exposed and likely targeted by acetylation. Acetylation of K609 blocks the adenylation reaction and stops the synthesis of acyl-AMP intermediates. Increased levels of AMP in the cell indirectly lead to lower levels of ATP synthesis, and this is the reason this enzyme is tightly regulated by reversible lysine acetylation. Abbreviations: Acs, acetyl-CoA synthetase; bGNAT, bacterial Gcn5 histone N-acetyltransferase; Nm, nicotinamide; O-AADPR, O-acetyl-ADP-ribose; PPi, pyrophosphate.