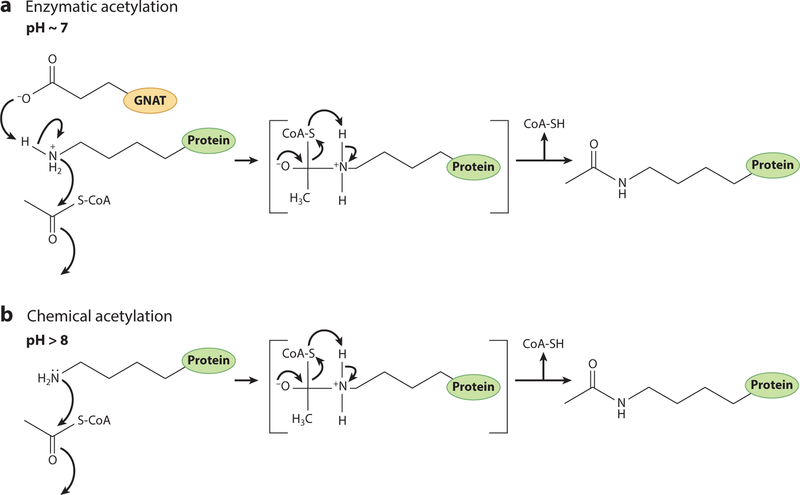
Figure 5.
Reaction mechanism of protein acetylation. (a) Bacterial Gcn5 histone N-acetyltransferase (bGNAT)-mediated protein acetylation involves a catalytic glutamate residue within the bGNAT core domain. The pKa of glutamate is 4.25, and at cellular pH (~7), the side chain is deprotonated. This allows the glutamate residue to act as a base that facilitates a water-mediated proton abstraction from the lysyl residue (pKa = 10.53) of the target protein. The epsilon amino group of the lysyl residue then undergoes a nucleophilic attack on the carbonyl carbon of the acetyl moiety of CoA, leading to the formation of an acetylated lysyl side chain. (b) Under high pH conditions, a larger portion of lysyl residues are deprotonated, bypassing the need for a catalytic proton abstraction from the side chain. Adapted with permission from Reference 25.