Figure 1.
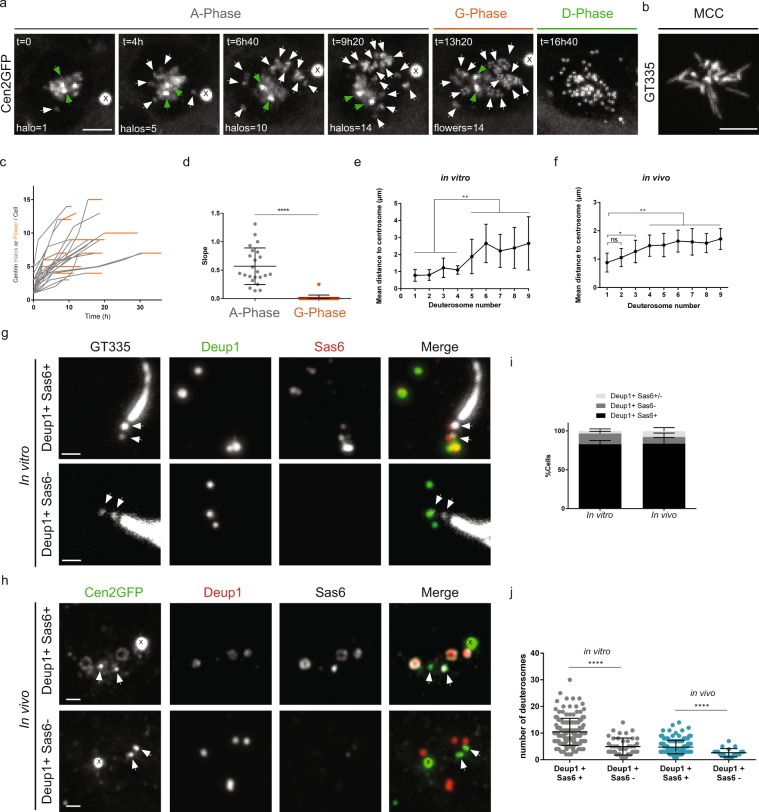
Centriole amplification proceeds sequentially and arises from the centrosomal region. (a) Time lapse sequences of a Cen2GFP ependymal progenitor undergoing the different stages (A-Phase, G-Phase and D-Phase) of centriole amplification. Early A-Phase is characterized by Cen2GFP cloud surrounding centrosomal centrioles and the first visible halos in the nearby cytoplasm. As amplification progresses, the number of halos increases and they localize throughout the cytoplasm. In G-Phase, the final number of halos is reached, Cen2GFP halos transform into flowers where procentrioles are becoming visible. In D-Phase, procentrioles individualize. White arrows indicate centrin halos or flowers. Green arrows indicate centrosomal centrioles. (b) Cilia immunostaining with GT335 antibody of a Cen2GFP ependymal MCC at the end of a time lapse experiment. (c) Number of centrin halos (Gray) or flowers (Orange) during time lapse experiments in Cen2GFP ependymal progenitors (Δt = 40 min, n = 23 cells). Halos or flowers are sometimes masked because of their backward movements toward the centrosomal region. Consequently, they have been counted during expansion phases when each single structure is clearly visible by 3D monitoring. (d) Trend line slopes corresponding to each cell observed in c in A-Phase and G-phase. (e,f) Mean distances of deuterosomes to the centrosome depending on the number of deuterosomes in the cell in vitro (e, n = 67 cells), and in vivo (f, n = 134 cells). Deuterosomes were counted when positive for Deup1 and Sas6 stainings. (g,h) Immunostaining of cells in A-phase with loaded (Deup1+/Sas6+) or unloaded (Deup1+/Sas6−) deuterosomes, in vitro (g), and in vivo (h). Arrows indicate centrosomal centrioles. (i) Quantification of cells with loaded (Deup1+/Sas6+), partially loaded (Deup1+/Sas6+/−) or unloaded deuterosomes (Deup1+/Sas6−) in vitro (n = 128 cells) and in vivo (n = 279 cells). (j) Quantification of the number of deuterosomes depending on the loading status of deuterosomes in vitro and in vivo (n = 196 cells in vitro, n = 165 cells in vivo). « X » indicates GFP aggregates. Scale bars: (a,b) 5 µm; (g,h) 1 µm.