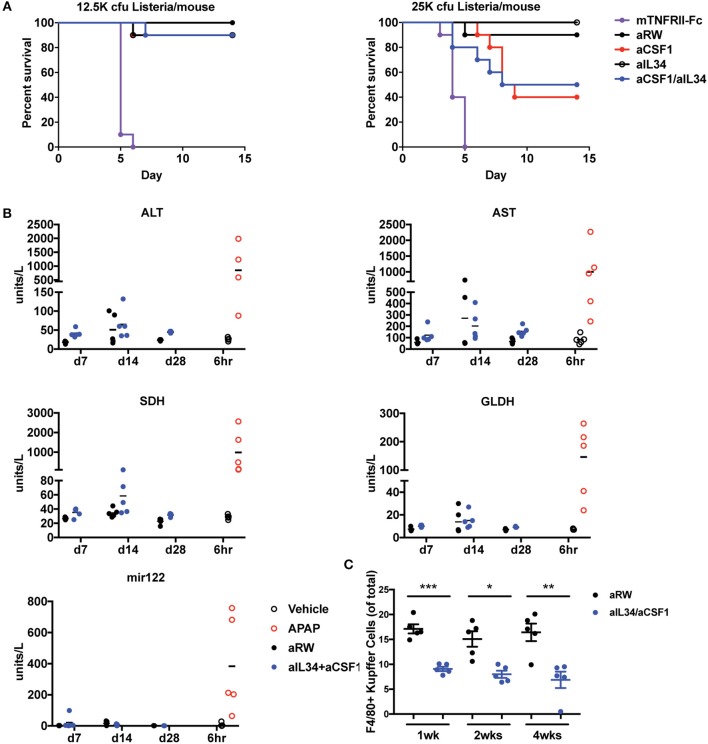
Figure 5.
Assessment of infection and liver toxicity risks with aIL34 and/or aCSF1. (A) Infection risk: Pre-treatment with aCSF1, aIL34, or aCSF1/aIL34 antibodies prior to L. monocytogenes infection resulted in an increase in survival rate compared to TNFRII-Fc. C57BL6 female mice (n = 10/group) were treated with aRW, aCSF1, aIL34, aCSF1/aIL34, or mTNFRII-Fc starting 2 days prior to infection. Mice were infected via intravenous route with two doses of L. monocytogenes [12.5K or 25K colony-forming units (cfu)/mouse] and animals were monitored for 14 days. (B) B6C3F1 mice were treated with aRW or a-CSF1/a-IL34 antibodies for 7, 14, or 28 days (n = 5/group/time point). Following high-dose APAP (1,200 mg/kg APAP for 6 h), increased ALT, AST, SDH, GLDH, and miR-122 values were observed. Treatment with a-CSF1/a-IL34 antibodies resulted in mild to moderate increases in liver injury biomarkers ALT and AST compared to concurrent anti-RW control values with no significant changes in liver injury biomarkers miR122 or GLDH. Following dosing, animals were necropsied, serum or plasma was collected for liver biomarker analysis, and liver tissue was processed for microscopic evaluation or enumeration of Kupffer cells as described in Materials and Methods. (C) Analysis of liver Kupffer cell numbers using F4/80+ IHC. Data in C are displayed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.