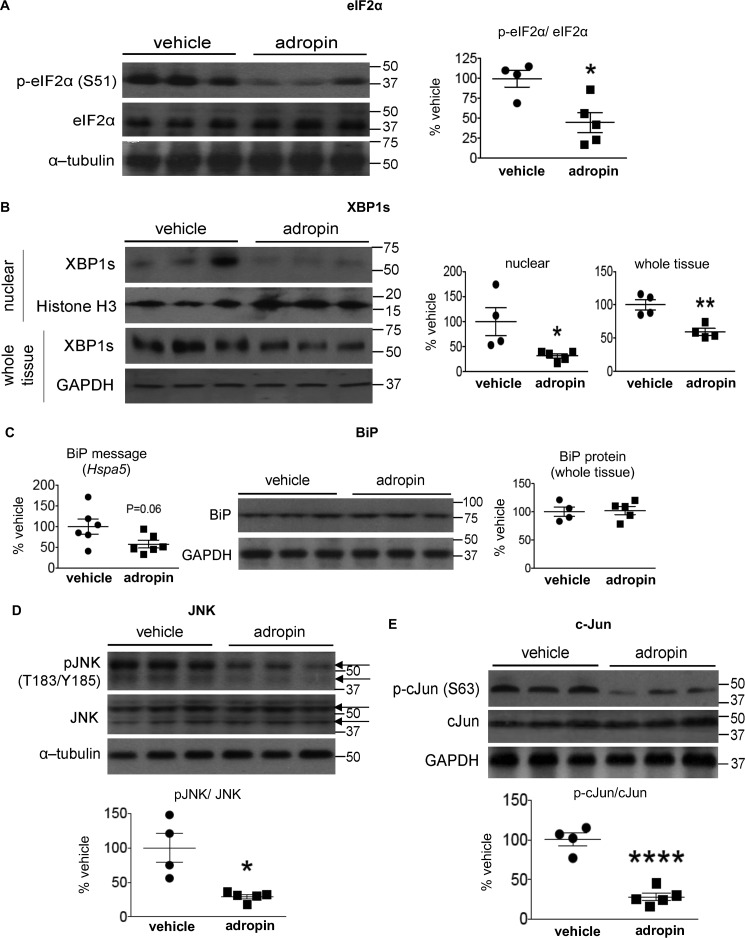
Figure 4.
Adropin34–76 treatment alleviated ER stress responses and diminished JNK signaling in the liver. A and B, the phosphorylation levels of Ser51 in eIF2α and total eIF2α levels in whole-tissue lysates (A) and the levels of XBP-1s in nuclear lysates (n = 4–5) and whole-tissue lysates (n = 4) (B) were determined by Western blotting (n = 4–5). In A, α-tubulin was used as the loading control. In B, histone H3 was used as the loading control for nuclear XBP1s, and GAPDH was used as the loading control for whole-tissue XBP1s. C, BiP message (Hspa5) levels (n = 6) and protein levels in whole-tissue lysates (n = 4–5) were determined by real-time RT-PCR and Western blotting, respectively. In Western blotting, GAPDH was used as the loading control for BiP. D and E, the phosphorylation levels of Thr183/Tyr185 in JNK and total JNK levels (n = 4–5) (arrows indicating JNK splice isoforms) (D) and the phosphorylation levels of Ser63 in c-Jun and total c-Jun levels (n = 4–5) in whole-tissue lysates (E) were determined by Western blotting (n = 4–5). In D, α-tubulin was used as the loading control. The same α-tubulin band was used as the loading control for the blot of whole-tissue IP3R1 (Fig. 7). In E, GAPDH was used as the loading control. The same GAPDH band was used as the loading control for the blot of total IRS2 (Fig. 1B) and the blots of pCREB (Ser133) and total CREB (Fig. 8B). *, p ≤ 0.05; ****, p < 0.0001, adropin versus vehicle. Error bars, S.E.