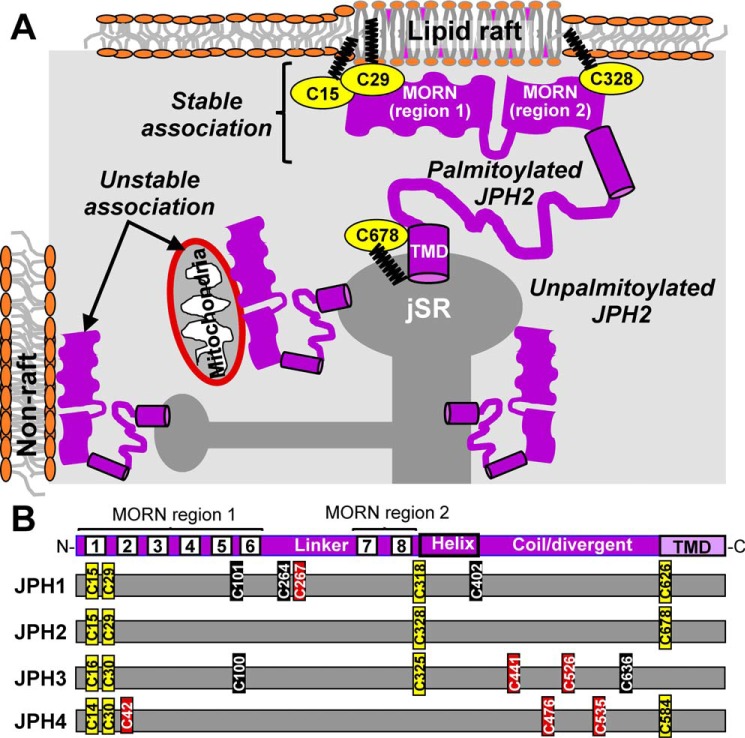
Figure 9.
A, working hypothesis for how palmitoylation directs JPH2 to SR/ER–PM junctions. Unpalmitoylated JPH2 in SR/ER has its MORN motifs exposed to the cytoplasm and may form unstable/transient associations with nonraft domains in plasma membrane or organelle (e.g. mitochondria). Palmitoylation of JPH2 promotes its binding to lipid–raft domains in PM, and binding to lipid–raft domains protects JPH2 from depalmitoylation. This synergism creates a stable association between palmitoylated JPH2 and PM. Palmitoylation of Cys-678 stabilizes JPH2 anchor in SR/ER membrane. B, Cys residues present in JPH1 (human sequence, accession no. Q9HDC5.2), JPH2 (Q9BR39.2), JPH3 (Q8WXH2.2), and JPH4 (NP_001139500.1) are marked relative to the shared JPH structural domains (top) and color-coded based on sequence alignment with JPH2 (yellow), high score (brown), or low score (black) as S-palmitoylation sites. Sequence alignment of JPH1–4 was done using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (32, 33) (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.), and prediction of Cys residues as potential S-palmitoylation sites was done using CSS-Palm 4.0 (http://csspalm.biocuckoo.org/online.php (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.)).