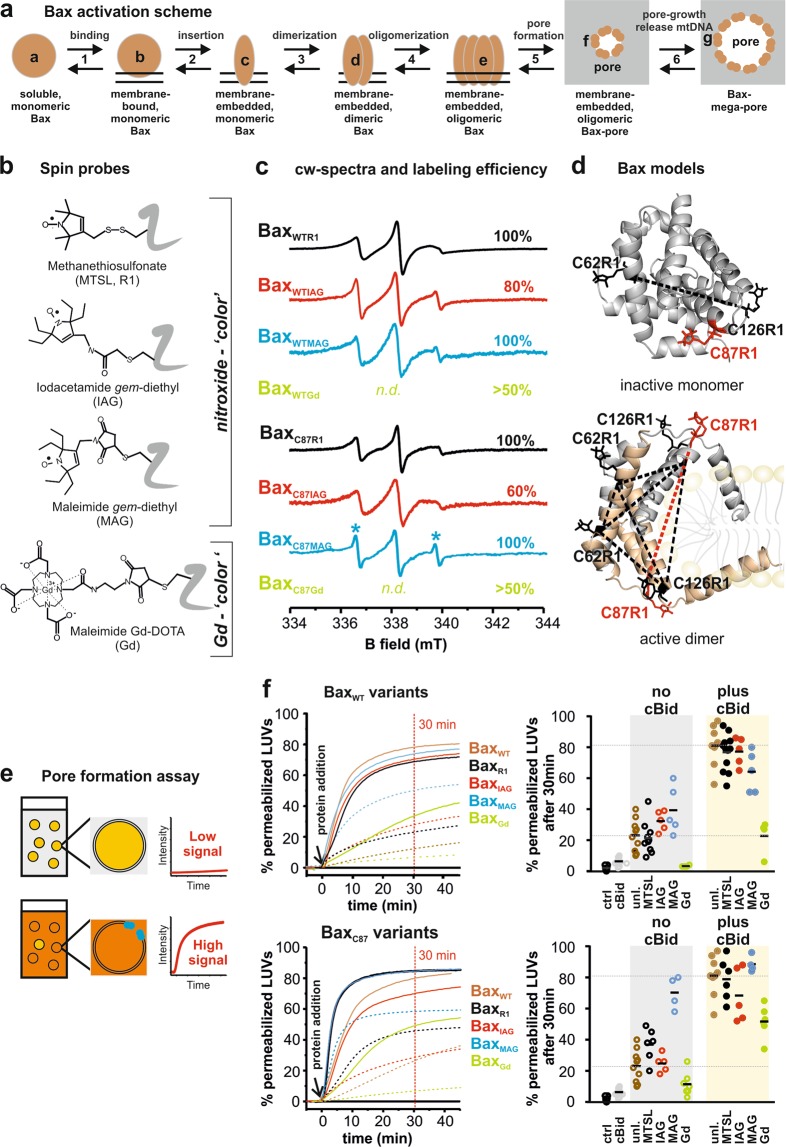
Figure 1.
Bax activation scheme, spin labeling and activity assays. (a) Sketch of conformational changes of Bax during activation. The protein exists at least in seven conformational states (a-g). Soluble monomeric Bax (state “a”) can transiently interact with the mitochondrial outer membrane MOM (“b”). Upon interaction with an activator-type BH3-only protein membrane insertion is induced (“c”)20,22 followed by the formation of homo-dimers (“d”) that assemble into higher order oligomers10,18,22,24 (“e”). The oligomers form a toroidal pore in the MOM allowing the release of Cyt c39,59 (“f”). These pores can grow to “mega-pores” able to release mitochondrial DNA14,17. (b) The four spin labels used in this study, targeting cysteine residues. (c) Room temperature cw spectra of the nitroxide-labeled proteins, with the degree of labeling (spin/cysteine ratio) indicated in %. Asterisks indicate residual free label. For Gd-labeled Bax see Supp. Fig. 2. (d) Structural models of monomeric12 and dimeric active Bax18 with one MTSL (R1) label attached per site using the software MMM55. Intra- and inter- monomer distances are highlighted by dotted lines (black for the wild type variants, red for the C87 variants). (e) Graphical scheme of the assay used to address Bax-induced pore formation. (f) Kinetic curves of pore formation (left panels) and data points after 30 min (right panels) for the BaxWT variants (upper panels) and for the BaxC87 variants (lower panels), both compared to unlabeled BaxWT. Dotted lines: Bax alone (50 nM) with vesicles; solid lines: 50 nM Bax with 50 nM cBid and vesicles. Note that the maximally reachable fraction of permeabilized vesicles varies for different experiments, depending on the lipid preparation (100% permeabilization was detected by adding detergent).