Abstract
BACKGROUND
Obstructive colorectal cancer (OCC) is always accompanied by severe complications, and the optimal strategy for patients with OCC remains undetermined. Different from emergency surgery (ES), self-expandable metal stents (SEMS) as a bridge to surgery (BTS), could increase the likelihood of primary anastomosis. However, the stent failure and related complications might give rise to a high recurrence rate. Few studies have focused on the indications for either method, and the relationship between preoperative inflammation indexes and the prognosis of OCC is still underestimated.
AIM
To explore the indications for ES and BTS in OCCs based on preoperative inflammation indexes.
METHODS
One hundred and twenty-eight patients who underwent ES or BTS from 2008 to 2015 were enrolled. Receiver operating characteristic (ROC) curve analysis was used to define the optimal preoperative inflammation index and its cutoff point. Kaplan–Meier analyses and Cox proportional hazards models were applied to assess the association between the preoperative inflammation indexes and the survival outcomes [overall survival (OS) and disease-free survival (DFS)]. Stratification analysis was performed to identify the subgroups that would benefit from ES or BTS.
RESULTS
OS and DFS were comparable between the ES and BTS groups (P > 0.05). ROC curve analysis showed derived neutrophil-to-lymphocyte ratio (dNLR) as the optimal biomarker for the prediction of DFS in ES (P < 0.05). Lymphocyte-to-monocyte ratio (LMR) was recommended for BTS with regard to OS and DFS (P < 0.05). dNLR was related to stoma construction (P = 0.001), pneumonia (P = 0.054), and DFS (P = 0.009) in ES. LMR was closely related to lymph node invasion (LVI) (P = 0.009), OS (P = 0.020), and DFS (P = 0.046) in the BTS group. dNLR was an independent risk factor for ES in both OS (P = 0.032) and DFS (P = 0.016). LMR affected OS (P = 0.053) and DFS (P = 0.052) in the BTS group. LMR could differentiate the OS between the ES and BTS groups (P < 0.05).
CONCLUSION
Preoperative dNLR and LMR could predict OS and DFS in patients undergoing ES and BTS, respectively. For OCC, as the potential benefit group, patients with a low LMR might be preferred for BTS via SEMS insertion.
Keywords: Inflammation indexes, Emergency surgery, Self-expanding metal stent insertion as a bridge to surgery, Obstructive colorectal cancers
Core tip: As a supplement to recent guidelines, this manuscript demonstrates that lymphocyte-to-monocyte ratio could effectively differentiates the survival outcome between self-expanding metal stenting and emergency surgery in patients with obstructive colon cancer. Self-expanding metal stents might be preferred to the “potential benefit group” that with a low preoperative lymphocyte-to-monocyte ratio (<1.67).
INTRODUCTION
Although several studies have been implemented in the screening for colorectal cancer, approximately 8%-29% of patients are diagnosed with obstructive colorectal cancer (OCC) as the first symptom[1,2]. Emergency surgery (ES) with or without stoma construction and self-expandable metal stent (SEMS) insertion as a bridge to surgery (BTS) are the current methods for OCC[3]. A BTS is preferred for symptomatic OCC due to effective decompression, better preoperative nutritional preparation, an improvement in the immunological reaction, and a lower incidence of stoma creation[4,5]. However, the enhancement of tumor dissemination and early recurrence reported by some studies hinder the usage of a self-expandable metal stent in OCC[6,7]. Despite this, there is still no common consensus. Several predictive models on the prognostic outcome of OCC, including ASA, age, Duck’s stage, and prognostic nutritional index, have been established[8,9], but few focus on the inflammation index[10].
The inflammatory response plays a dual role in the development of a tumor. On one hand, a chronic inflammatory response triggers the local accumulation of monocytes, platelets, and neutrophils, which secrete cytokines and inflammatory factors to induce tumor angiogenesis and metastasis. On the other hand, increasing monocytes and lymphatic cells would enhance the resistance against tumor invasion[11]. Increasing evidence shows that an elevated neutrophil-to-lymphocyte ratio (NLR) is closely related to a poor prognosis in ovarian cancer, cholangiocarcinoma, and elective colorectal cancer (CRC)[12-14]. The overexpression of circulating derived NLR, an effective biomarker for the diagnosis of early pancreatic cancer[15], was accompanied by increasing distal organ invasion in metastatic CRC[16]. An elevated preoperative lymphocyte-to-monocyte ratio (LMR), as a superior existing biomarker, was positively correlated with the survival outcomes of patients with resectable CRC and presented better overall survival[17]. Other inflammatory indexes, such as the platelet-to-lymphocyte ratio (PLR)[14] and systemic immune inflammation index (SII)[18], have also been studied in the exploration of optimal predictive models for tumor recurrence.
Different from the acute inflammatory response in patients undergoing ES, the alleviation of bowel obstruction after successful SEMS insertion in patients undergoing BTS would elicit a better immunological reaction and nutritional support, which might change the predictive factors for prognosis between the two groups. Preoperative inflammation indexes might favor patient selection and the establishment of a valid predictive model for the prognosis of OCC. In this study, we compared different inflammation indexes and other clinicopathological factors to evaluate the potential indications for ES and BTS for OCC.
MATERIALS AND METHODS
Patient population
All patients (n = 128) who underwent surgery for OCC at the Department of Emergency Surgery of Fujian Medical University Union Hospital from January 2008 to October 2015 were included in this study. Data from the patients’ records were retrospectively collected and evaluated. The Institutional Review Board of Fujian Medical Union Hospital approved the study protocol. All patients provided informed consent for surgery. Patients were divided into an ES group and a BTS group based on the grade of bowel obstruction and families’ choices. For incomplete obstruction, ES was preferred as the first choice. For complete obstruction, once patients who refused to accept SEMS insertion or failed in SEMS insertion, they would accept ES with intraoperative decompression.
Classification criteria
Patients who manifested with bowel obstruction were enrolled in this study. All diagnoses of OCC were confirmed by both emergency abdominal computed tomography (CT) and a pathological examination. The exclusion criteria were as follows: (1) Patients who rejected surgery or were diagnosed with acute peritonitis or perforation; (2) Patients with severe infection, hematological diseases, or an immunological deficit; and (3) Patients who received preoperative adjuvant chemotherapy, radiotherapy, or immunotherapy.
Surgical protocols
For left-side OCC, we performed intraoperative lavage or manual decompression for better bowel preparation, and these protocols have been previously depicted. For right-side OCC, radical dissection with one-stage anastomosis was performed[19].
SEMS with BTS
Stent insertion was performed by an endoscopist who had experienced over 400 endoscopic retrograde cholangiopancreatography (ERCP) procedures. Bridge to elective surgery was performed, once the stent was so successfully inserted that the intestinal obstruction completely relieved. Otherwise, ES was immediately performed.
Definition of variants
The neutrophil, lymphocyte, monocyte, and platelet counts from the peripheral blood tests and the inflammation indexes dependent on these factors were performed before surgery (e.g., NLR, dNLR, LMR, PLR, and SII) and stent insertion (e.g., NLR-pre, dNLR-pre, LMR-pre, PLR-pre, and SII-pre). The methods for the calculation of NLR, dNLR, LMR, and PLR have been described in previous studies[13]. The SII was calculated as (platelet count × neutrophil count)/lymphocyte count[18]. The cutoff point and the area under the curve (AUC) value of each inflammation index for the prediction of OS and DFS were determined with X-tile 3.6.1 software (Yale University, New Haven, CT, United States)[20]. According to the cutoff point, patients were divided into low-ratio and high-ratio groups for further analysis.
According to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition)[21], we classified the tumor pathological stage. Comorbidities were defined as hypertension, diabetes mellitus, and single and multiple organ dysfunction. The degree of obstructive symptoms was divided into five grades, termed as The ColoRectal Obstruction Scoring System (CROSS)[22]. According to the Clavien-Dindo classification system[23,24], we classified the perioperative complications into five grades.
Statistical analysis
Qualitative variables were compared by the χ2 test or Fisher’s exact test, and quantitative variables were compared via t-tests. Through Kaplan-Meier analysis, the 3-year OS and 3-year DFS were calculated. A Cox proportional hazards regression model was built to identify the independent risk factors for 3-year DFS and 3-year OS. Stratification analysis was used to compare the differences between subgroups. All P-values less than 0.05 were considered statistically significant. All statistical analyses and graphs were generated using SPSS 23.0 software.
RESULTS
Baseline characteristics
There were 128 patients enrolled in this study, who were divided into an ES group (n = 90) and a BTS group (n = 38), with similar age and sex ratios between the groups (P > 0.05). The average tumor size was 6.88 ± 2.68 cm in the BTS group, with a higher proportion of tumors located on the left side of the colon (73.70% vs 41.10%, P = 0.005), and was much larger than the tumor size in the ES group (5.76 ± 2.12 cm, P = 0.015). Moreover, the obstructive symptoms were more severe in the BTS group than in the ES group (Grade 0-I, 97.40% vs 68.50%, P = 0.001), as presented in Table 1. The remaining characteristic factors, including BMI, abdominal surgery history, comorbidities, ASA grade, pTNM stage, histological features, and the ratio of chemotherapy were similar between the ES and BTS groups (P > 0.05).
Table 1.
Comparison of clinicopathological characteristics between emergency surgery and bridge to surgery groups
| Characteristic | ES group (n = 90) | BTS group (n = 38) | P-value |
| Age (yr) | 61.58 ± 14.84 | 63.21 ± 13.55 | 0.561 |
| Female/Male, (%) | 31 (34.40)/59 (65.60) | 15 (39.50)/23 (60.50) | 0.588 |
| Size, (cm) | 5.76 ± 2.12 | 6.88 ± 2.68 | 0.015 |
| BMI, (kg/m2) | 21.76 ± 2.42 | 22.20 ± 3.20 | 0.411 |
| Cross score, (%) | 0.001 | ||
| 0 | 21 (23.60) | 21 (55.30) | |
| 1 | 40 (44.90) | 16 (42.10) | |
| 2 | 17 (19.10) | 1 (2.60) | |
| 3 | 10 (11.20) | 0 (0.00) | |
| 4 | 1 (0.80) | 0 (0.00) | |
| ASH (+)/(-), (%) | 17 (18.90)/73 (81.10) | 10 (26.30)/28 (73.70) | 0.347 |
| Comorbidities (+)/(-), (%) | 37 (41.10)/53 (58.90) | 21 (55.30)/17 (44.70) | 0.142 |
| ASA grade, (%) | 0.299 | ||
| I | 2 (2.20) | 3 (7.90) | |
| II | 63 (70.00) | 28 (73.70) | |
| ≥III | 25 (27.80) | 7 (18.40) | |
| Location, (%) | 0.005 | ||
| Right-side colon | 13 (14.40) | 1 (2.60) | |
| Transverse colon | 30 (33.30) | 5 (13.20) | |
| Left-side colon | 37 (41.10) | 28 (73.70) | |
| Rectum | 10 (11.10) | 4 (10.50) | |
| pTNM stage, (%) | 0.186 | ||
| I | 4 (4.40) | 0 (0.00) | |
| II | 23 (25.60) | 9 (23.70) | |
| III | 44 (48.90) | 25 (65.80) | |
| IV | 19 (21.10) | 4 (10.50) | |
| T stage, (%) | 0.186 | ||
| T1 | 4 (4.40) | 0 (0.00) | |
| T2 | 23 (25.60) | 9 (23.70) | |
| T3 | 44 (48.90) | 25 (65.80) | |
| T4 | 19 (21.10) | 4 (10.50) | |
| N stage, (%) | 0.471 | ||
| N0 | 31 (34.40) | 9 (23.70) | |
| N1 | 35 (38.90) | 18 (47.40) | |
| N2 | 24 (26.70) | 11 (28.90) | |
| M stage, (%) | 0.292 | ||
| M0 | 71 (78.9) | 33 (86.8) | |
| M1 | 19 (21.1) | 5 (13.2) | |
| Histological features, (%) | 0.308 | ||
| Well differentiated | 3 (2.30) | 0 (0.00) | |
| Moderately differentiated | 61 (67.80) | 30 (78.90) | |
| Poorly differentiated | 26 (28.90) | 8 (21.10) | |
| LVI (+)/(-), (%) | 15 (16.70)/75 (83.30) | 14(36.80)/24(63.20) | 0.013 |
| WBC, (10^9) | 8.99 ± 5.10 | 7.57 ± 2.61 | 0.042 |
| NLR, (ratio) | 7.11 ± 6.72 | 4.88 ± 3.02 | 0.012 |
| dNLR, (ratio) | 1.66 ± 0.41 | 1.67 ± 0.27 | 0.756 |
| PLR, (ratio) | 245.61 ± 144.17 | 229.98 ± 122.38 | 0.562 |
| LMR, (ratio) | 2.84 ± 2.43 | 2.34 ± 1.19 | 0.127 |
| SII, (ratio) | 1969.03 ± 2316.10 | 1235.74 ± 849.53 | 0.011 |
| WBC-pre, (10^9) | 9.18 ± 5.13 | 8.56 ± 3.44 | 0.434 |
| NLR-pre, (ratio) | 7.62 ± 6.97 | 6.05 ± 3.03 | 0.084 |
| dNLR-pre, (ratio) | 1.65 ± 0.41 | 1.68 ± 0.45 | 0.652 |
| PLR-pre, (ratio) | 263.98 ± 161.96 | 270.89 ± 171.35 | 0.830 |
| LMR-pre, (ratio) | 2.77 ± 2.32 | 2.38 ± 1.66 | 0.354 |
| SII-pre, (ratio) | 2186.46 ± 2474.96 | 1712.60 ± 1157.32 | 0.149 |
| CEA, (ng/mL) | 30.19 ± 120.54 | 17.88 ± 27.47 | 0.541 |
| Chemotherapy (+)/(-), (%) | 62 (68.90)/28 (31.10) | 20 (52.60)/18 (47.40) | 0.080 |
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; ASH: Abdominal surgery history; WBC: White blood cells; dNLR: Derived neutrophil–to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune inflammation index; LMR: Lymphocyte-to-monocyte ratio; Cross: Colorectal obstruction scoring system; LVI: Lymphovascular invasion. P < 0.05 was considered statistically significant.
Outcome comparison between the ES and BTS groups
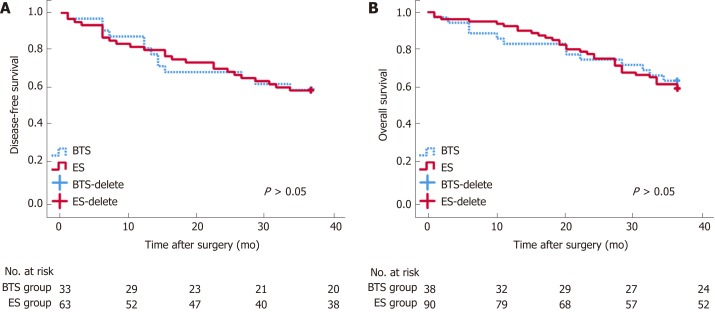
The blood loss in the BTS group was lower than that in the ES group (133.68 ± 95.76 mL vs 177.30 ± 134.37 mL, P = 0.072), with similar gastrointestinal recovery and postoperative complications (P > 0.05) (Table 2). Analogical survival outcomes including 3-year OS (30.10 ± 9.64 mo vs 29.41 ± 11.33 mo, P = 0.732) and 3-year DFS (27.59 ± 12.19 mo vs 27.48 12.17 mo, P = 0.969) were compared between the ES and BTS groups, and are plotted in Figure 1.
Table 2.
Comparison of short-term and long-term outcomes between emergency surgery and bridge to surgery groups
| Characteristic | ES group (n = 90) | BTS group (n = 38) | P-value |
| Surgical time, (min) | 217.89 ± 60.69 | 204.64 ± 66.13 | 0.275 |
| Blood loss, (mL) | 177.30 ± 134.37 | 133.68 ± 95.76 | 0.072 |
| Number of LNs | 19.51 ± 9.47 | 21.45 ± 8.29 | 0.276 |
| Time to flatus, (d) | 3.88 ± 1.65 | 3.61 ± 1.15 | 0.359 |
| Time to semi-fluid, (d) | 8.62 ± 3.22 | 8.64 ± 3.96 | 0.738 |
| Total hospital-stay, (d) | 22.17 ± 12.48 | 22.34 ± 7.78 | 0.936 |
| Stoma construction, n (%) | 20 (22.20) | 8 (21.10) | 0.884 |
| CD classification system, n (%) | 0.547 | ||
| Grade I | 0 (0.00) | 2 (2.20) | |
| Grade II | 44 (48.90) | 16 (42.10) | |
| Grade III | 13 (14.40) | 5 (13.20) | |
| Grade IV | 9 (10.00) | 2 (5.30) | |
| Grade V | 1 (2.60) | 1 (1.10) | |
| Pneumonia, n (%) | 18 (20.00) | 8 (21.10) | 0.892 |
| Incision infection, n (%) | 16 (17.80) | 5 (13.20) | 0.519 |
| ICU intervention, n (%) | 8 (8.90) | 1 (2.60) | 0.192 |
| Leakage, n (%) | 3 (3.30) | 1 (2.60) | 0.658 |
| Sepsis, n (%) | 3 (3.30) | 1 (2.60) | 0.658 |
| SAE, n (%) | 23 (25.60) | 8 (21.10) | 0.587 |
| 30 d-mortality, n (%) | 1 (1.10) | 1 (2.60) | 0.507 |
| 36-OS time, (mo) | 30.10 ± 9.64 | 29.41 ± 11.33 | 0.732 |
| 36-DFS time, (mo) | 27.59 ± 12.19 | 27.48 ± 12.17 | 0.969 |
LN: Lymph node; SEMS: Self-expanding metal stents; BTS: Bridge to surgery; SAE: Severe adverse effects. P < 0.05 was considered statistically significant.
Figure 1.
Long-term survival analysis between emergency surgery and bridge to surgery groups. Disease-free survival (DFS, A) and overall survival (OS, B) after surgery seemed similar between the bridge to surgery (BTS) and emergency surgery (ES) groups.
Predictive values and cutoff points of different inflammation indexes
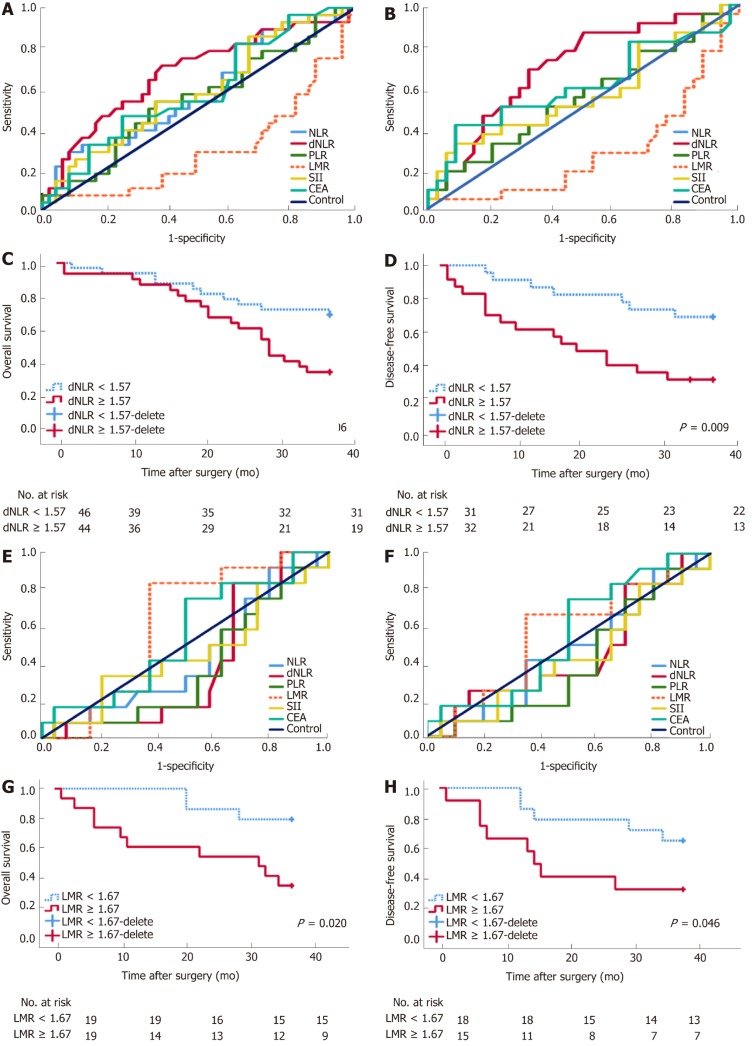
A decreasing tendency was observed for WBC (8.56 × 109 ± 3.44 × 109), NLR (4.88 ± 3.02), and SII (1235.74 ± 849.53) in the BTS group after SEMS insertion, compared with the WBC (7.57 × 109 ± 2.61 × 109), NLR (6.05 ± 3.03), and SII (1712.60 ± 1157. 32) before SEMS insertion (P < 0.05), as presented in Table 1. Different inflammation indexes were analyzed between the ES and BTS groups. As a result, dNLR was preferred as a prognostic biomarker for the ES group since it had the highest AUC for 3-year OS (0.679, 95%CI: 0.551-0.808) and 3-year DFS (0.679, 95%CI: 0.551-0.808); the cutoff point value was 1.57. Conversely, based on the highest AUC for 3-year OS (0.611, 95%CI: 0.424-0.798) and 3-year DFS (0.571, 95%CI: 0.366-0.776), the LMR was recommended as a prognostic biomarker for the BTS group, with 1.67 as its cutoff point. These data are depicted in Table 3 and plotted in Figure 2.
Table 3.
Receiver operating characteristic curve analysis of long-term survival of emergency surgery and bridge to surgery groups
| Group | Characteristic |
3-year OS |
3-year DFS |
||||
| Cutoff point | AUC | 95%CI | Cutoff point | AUC | 95%CI | ||
| ES | NLR | 19.3 | 0.582 | 0.446-0.718 | 19.3 | 0.565 | 0.407-0.723 |
| dNLR | 2.02 | 0.679 | 0.551-0.808 | 1.57 | 0.696 | 0.554-0.837 | |
| PLR | 155 | 0.550 | 0.414-0.686 | 317 | 0.549 | 0.392-0.707 | |
| SII | 3645 | 0.587 | 0.454-0.721 | 3645 | 0.564 | 0.403-0.726 | |
| CEA | 6.7 | 0.591 | 0.458-0.724 | 11.2 | 0.604 | 0.442-0.766 | |
| BTS | LMR | 1.67 | 0.611 | 0.424-0.798 | 1.67 | 0.571 | 0.366-0.776 |
| CEA | 7.6 | 0.549 | 0.350-0.747 | 5.5 | 0.552 | 0.348-0.756 | |
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; AUC: Area under the receiver operating characteristic curve; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet–to-lymphocyte ratio; SII: Systemic immune inflammation index; LMR: Lymphocyte-to-monocyte ratio; CEA: Carcino-embryonic antigen. P < 0.05 was considered statistically significant.
Figure 2.
Receiver operating characteristic curve and long-term survival analysis of emergency surgery and bridge to surgery group. Derived neutrophil-to-lymphocyte ratio (dNLR) is preferred as a prognostic biomarker for the emergency surgery (ES) group with the highest area under receiver operating characteristic curve (AUC) for 3-year overall survival (OS) (0.679, 95%CI: 0.551-0.808) (A) and 3-year disease-free survival (DFS) (0.679, 95%CI: 0.551-0.808) (B), with a cutoff point value of 1.57. High-ratio grade of dNLR (≥1.57) was closely related to lower 3-year DFS (≥1.57 vs <1.57, 23.10 ± 13.85 mo vs 31.45 ± 9.35 mo, P = 0.009) in the ES group (D), but not with 3-year OS (C). Lymphocyte-to-monocyte ratio (LMR) was preferred as a prognostic biomarker for bridge to surgery (BTS) group with the highest AUC for 3-year OS (0.611, 95%CI: 0.424-0.798) (E) and 3-year DFS (0.571, 95%CI: 0.366-0.776) (F), with a cutoff point value of 1.67. High-ratio grade of LMR (≥1.67) was closely related to lower 3-year OS (≥1.67 vs <1.67, 23.10 ± 13.85 mo vs 33.78 ± 5.35 mo, P = 0.020) (G) and 3-year DFS (≥ 1.67 vs < 1.67, 22.67 ± 14.02 mo vs 31.50 ± 8.89 mo, P = 0.046) in the BTS group (H).
Clinical evaluation of different inflammation indexes
In Table 4, patients were divided into high-ratio and low-ratio grades based on the dNLR in the ES group and the LMR in the BTS group. A high-ratio grade of dNLR (≥ 1.57) was closely related to a higher proportion of tumors located on the left side of the colon and rectum (P = 0.007), and a higher incidence of stoma construction (P = 0.001) and postoperative pneumonia (P = 0.054), with a lower 3-year DFS (dNLR ≥ 1.57: 23.10 ± 13.85 mo vs dNLR < 1.57: 31.45 ± 9.35 mo, P = 0.009) in the ES group. Separately, a high-ratio grade of the LMR (≥ 1.67) in the BTS group showed more advanced lymphovascular metastasis (P = 0.072) and lymph node invasion (P = 0.009), with a lower 3-year OS (LMR ≥ 1.67: 25.26 ± 13.88 mo vs LMR < 1.67: 33.78 ± 5.35 mo, P = 0.020) and 3-year DFS (LMR ≥ 1.67: 22.67 ± 14.02 mo vs LMR < 1.67: 31.50 ± 8.89 mo, P = 0.046). The dNLR was the only independent risk factor in the ES group both for 3-year OS (HR = 2.34, 95%CI: 1.08-5.07, P = 0.032) and 3-year DFS (HR = 3.02, 95%CI: 1.23-7.42, P = 0.016). In contrast, the status of LVI (HR = 3.52, 95%CI: 1.03-12.02, P = 0.045) and the LMR (HR = 4.57, 95%CI: 0.98-21.38, P = 0.053) significantly affected the 3-year OS in the BTS group. Only the LMR was an independent risk factor for 3-year DFS (HR = 3.11, 95%CI: 1.13-8.54, P = 0.052) in the BTS group, as shown in Tables 5 and 6 and Figure 2.
Table 4.
Comparison of clinicopathological features between high-ratio and low-ratio grades in both emergency surgery and bridge to surgery groups
| Characteristic |
ES group (n = 86) |
BTS group (n = 38) |
||||
| dNLR ≥ 1.57 | dNLR < 1.57 | P-value | LMR ≥ 1.67 | LMR < 1.67 | P-value | |
| Cross score, (%) | 0.738 | 0.378 | ||||
| 0 | 11 (27.5) | 10 (21.7) | 10 (50.0) | 11 (61.1) | ||
| 1 | 16 (40.0) | 22 (47.8) | 10 (50.0) | 6 (33.3) | ||
| 2 | 8 (20.0) | 8 (17.4) | 0 (0.0) | 1 (5.6) | ||
| 3 | 4 (10.0) | 6 (13.0) | ||||
| 4 | 1 (2.5) | 0 (0.0) | ||||
| ASH (+)/(-), (%) | 6 (15.0)/34 (85.0) | 10 (21.7)/36 (78.3) | 4 (20.0)/16 (80.0) | 6 (33.3)/12 (66.7) | ||
| Comorbidities (+)/(-), (%) | 20 (50.0)/20 (50.0) | 17 (37.0)/29 (63.0) | 9 (45.0)/11 (55.0) | 12 (66.7/)/6 (33.3) | ||
| ASA grade, (%) | 0.320 | 0.623 | ||||
| I | 0 (0.0) | 1 (1.6) | 1 (5.0) | 2 (11.1) | ||
| II | 13 (56.5) | 47 (74.6) | 16 (80.0) | 12 (66.7) | ||
| ≥III | 10 (43.5) | 15 (23.8) | 3 (15.0) | 4 (22.2) | ||
| Location, (%) | 0.007 | 0.523 | ||||
| Right-side colon | 2 (5.0) | 11 (23.9) | 1 (5.0) | 0 (0.00) | ||
| Transverse colon | 10 (25.0) | 19 (41.3) | 3 (15.0) | 2 (11.1) | ||
| Left-side colon | 21 (52.5) | 13 (28.3) | 13 (65.0) | 15 (83.3) | ||
| Rectum | 7 (17.5) | 3 (6.5) | 3 (15.0) | 1 (5.6) | ||
| pTNM stage, (%) | 0.141 | 0.592 | ||||
| I | 0 (0.0) | 4 (8.7) | - | - | ||
| II | 12 (30.0) | 10 (21.7) | 4 (20.0) | 5 (27.8) | ||
| III | 17 (42.5) | 24 (52.2) | 13 (65.0) | 12 (66.7) | ||
| IV | 11 (27.5) | 8 (17.4) | 3 (15.0) | 1 (5.6) | ||
| T stage, (%) | 0.141 | 0.592 | ||||
| T1 | 0 (0.0) | 4 (8.7) | - | - | ||
| T2 | 12 (30.0) | 10 (21.7) | 4 (20.0) | 5 (27.8) | ||
| T3 | 17 (42.5) | 24 (52.2) | 13 (65.0) | 12 (66.7) | ||
| T4 | 11 (27.5) | 8 (17.4) | 3 (15.0) | 1 (5.6) | ||
| N stage, (%) | 0.648 | 0.009 | ||||
| N0 | 16 (40.0) | 14 (30.4) | 4 (20.0) | 5 (27.8) | ||
| N1 | 14 (35.0) | 19 (41.3) | 6 (30.0) | 12 (66.7) | ||
| N2 | 10 (25.0) | 13 (28.3) | 10 (50.0) | 1 (5.6) | ||
| M stage, (%) | 0.260 | 0.552 | ||||
| M0 | 29 (72.5) | 38 (82.6) | 17 (85.0) | 16 (88.9) | ||
| M1 | 11 (27.5) | 8 (17.4) | 3 (15.0) | 2 (11.1) | ||
| Histological features, (%) | 0.605 | 0.411 | ||||
| Well differentiated | 1 (2.5) | 2 (4.3) | - | - | ||
| Moderately differentiated | 30 (75.0) | 30 (65.2) | 15 (75.0) | 15 (83.3) | ||
| Poorly differentiated | 9 (22.5) | 14 (30.4) | 5 (25.0) | 3 (16.7) | ||
| LVI (+)/(-), (%) | 9 (22.5)/31 (77.5) | 6 (13.0)/40 (87.0) | 0.249 | 10 (50.0)/10 (50.0) | 4 (22.2)/14 (77.8) | 0.076 |
| Stoma construction, (%) | 0.000 | 0.589 | ||||
| Stoma | 17 (42.5) | 3 (6.5) | 4 (20.0) | 4 (22.2) | ||
| None | 23(57.5) | 43 (93.5) | 16 (80.0) | 14 (77.8) | ||
| Pneumonia, (+)/(-), (%) | 12 (30.0)/28 (70.0) | 6 (13.0)/40 (87.0) | 0.054 | 2 (10.0)/18(90.0) | 6 (33.3)/12 (66.7) | 0.086 |
| Incision infection, (+)/(-), (%) | 8 (20.0)/32 (80.0) | 8 (17.4)/38 (82.6) | 0.486 | 2 (10.0)/18 (90.0) | 3 (16.7)/15 (83.3) | 0.448 |
| ICU intervention, (+)/(-), (%) | 5 (12.5)/35 (87.5) | 3 (6.5)/43 (93.5) | 0.281 | 1 (5.0)/19 (95.0) | 0 (0.0)/18 (100.0) | 0.526 |
| Leakage, (+)/(-), (%) | 1 (2.5)/39 (97.5) | 2 (4.3)/44 (95.7) | 0.553 | 0 (0.0)/20 (100.0) | 1 (5.6)/17 (94.4) | 0.474 |
| Sepsis, (+)/(-), (%) | 1 (2.5)/39 (97.5) | 2 (4.3)/44 (95.7) | 0.553 | 0 (0.0)/20 (100.0) | 1 (5.6)/17 (94.4) | 0.474 |
| SAE, (+)/(-), (%) | 10 (25.0)/30 (75.0) | 11 (23.9)/35 (76.1) | 0.907 | 5 (25.0)/15 (75.0) | 3 (16.7)/15 (83.3) | 0.411 |
| 30-day mortality, n (%) | 1 (2.5)/39 (97.5) | 0 (0.0)/46 (100.0) | 0.465 | 1 (5.0)/19 (95.0) | 0 (0.0)/18 (100.0) | 0.526 |
| 36-OS time, (months) | 28.05 ± 10.28 | 31.61 ± 9.16 | 0.106 | 25.26 ± 13.88 | 33.78 ± 5.35 | 0.020 |
| 36-DFS time, (months) | 23.10 ± 13.85 | 31.45 ± 9.35 | 0.009 | 22.67 ± 14.02 | 31.50 ± 8.89 | 0.046 |
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; Cross: Colorectal obstruction scoring system; ASH: Abdominal surgery history; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune inflammation index; LMR: Lymphocyte-to-monocyte ratio; LVI: Lymphovascular invasion; OS: Overall survival; DFS: Disease-free survival; ICU: Intense care unit; SAE: Severe adverse effects. P < 0.05 was considered statistically significant.
Table 5.
Univariate and multivariate analyses of risk factors for survival outcomes in both emergency surgery and bridge to surgery groups
| 3-year overall survival |
ES group (n = 90) |
BTS group (n = 38) |
||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Characteristic | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value |
| CEA (≥5 ng/mL vs <5 ng/mL) | 1.48 (0.70-3.11) | 0.303 | 2.53 (0.68-9.35) | 0.165 | ||||
| ASA (Grade ≥ III vs Grade < III) | 1.50 (0.72-3.11) | 0.277 | 1.64 (0.45-5.96) | 0.454 | ||||
| pT stage (pT3-4 vs pT1-2) | 1.66 (0.72-3.83) | 0.238 | 4.17 (1.09-15.95) | 0.037 | ||||
| pN stage (pN+ vs pN0) | 1.05 (0.51-2.19) | 0.887 | 5.02 (0.65-38.66) | 0.122 | ||||
| LVI (+) vs LVI (-) | 1.30 (0.53-3.15) | 0.568 | 3.78 (1.23-11.64) | 0.020 | 3.52 (1.03-12.02) | 0.045 | ||
| NLR ≥ 19.3 vs NLR < 19.3 | 2.98 (1.27-6.97) | 0.012 | ||||||
| dNLR ≥ 1.57 vs dNLR < 1.57 | 2.40 (1.12-5.13) | 0.024 | 2.34 (1.08-5.07) | 0.032 | ||||
| PLR ≥ 155 vs PLR < 155 | 1.83 (0.70-4.79) | 0.217 | ||||||
| SII ≥ 3645 vs SII < 3645 | 1.61 (0.71-3.61) | 0.252 | ||||||
| LMR ≥ 1.67 vs LMR < 1.67 | 4.09 (1.12-14.87) | 0.033 | 4.57 (0.98-21.38) | 0.053 | ||||
| Chemotherapy (+) vs (-) | 0.74 (0.36-1.51) | 0.402 | 1.43 (0.47-4.38) | 0.529 | ||||
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet–to-lymphocyte ratio; SII: Systemic immune inflammation index; LMR: Lymphocyte-to-monocyte ratio; LVI: Lymphovascular invasion. P < 0.05 was considered significant.
Table 6.
Univariate and multivariate analyses of risk factors for oncological outcomes in both emergency surgery and bridge to surgery groups
| 3-year disease-free survival |
ES group (n = 56) |
BTS group (n = 32) |
||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Characteristic | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value |
| CEA (≥ 5 ng/mL vs <5 ng/mL) | 1.71 (0.74-3.95) | 0.209 | 2.67 (0.72-9.90) | 0.141 | ||||
| ASA (Grade ≥ III vs Grade < III) | 0.890 (0.36-2.23) | 0.803 | 1.49 (0.41-5.43) | 0.542 | ||||
| pT stage (pT3-4 vs pT1-2) | 2.26 (0.85-6.02) | 0.104 | 2.48 (0.55-11.18) | 0.239 | ||||
| pN stage (pN+ vs pN0) | 1.48 (0.64-3.43) | 0.361 | 2.48 (0.55-11.18) | 0.239 | ||||
| LVI (+) vs LVI (-) | 2.92 (1.25-6.81) | 0.013 | 1.97 (0.66-5.88) | 0.224 | ||||
| NLR ≥ 19.3 vs NLR < 19.3 | 2.76 (1.02-7.45) | 0.046 | ||||||
| dNLR ≥ 1.57 vs dNLR < 1.57 | 2.85 (1.17-6.95) | 0.021 | 3.02(1.23-7.42) | 0.016 | ||||
| PLR ≥ 317 vs PLR < 317 | 1.55 (0.66-3.67) | 0.314 | ||||||
| SII ≥ 3645 vs SII < 3645 | 2.04 (0.86-4.83) | 0.104 | ||||||
| LMR ≥ 1.67 vs LMR < 1.67 | 2.54 (0.83-7.80) | 0.091 | 3.11 (1.13-8.54) | 0.052 | ||||
| Chemotherapy (+) vs (-) | 0.95 (0.41-2.19) | 0.896 | 1.44 (0.47-4.41) | 0.523 | ||||
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune inflammation index; LMR: Lymphocyte-to-monocyte ratio; LVI: Lymphovascular invasion. Chemotherapy (+), accept chemotherapy lately. Chemotherapy (-), refuse to chemotherapy lately. P < 0.05 was considered significant.
Selective choices based on inflammatory biomarkers
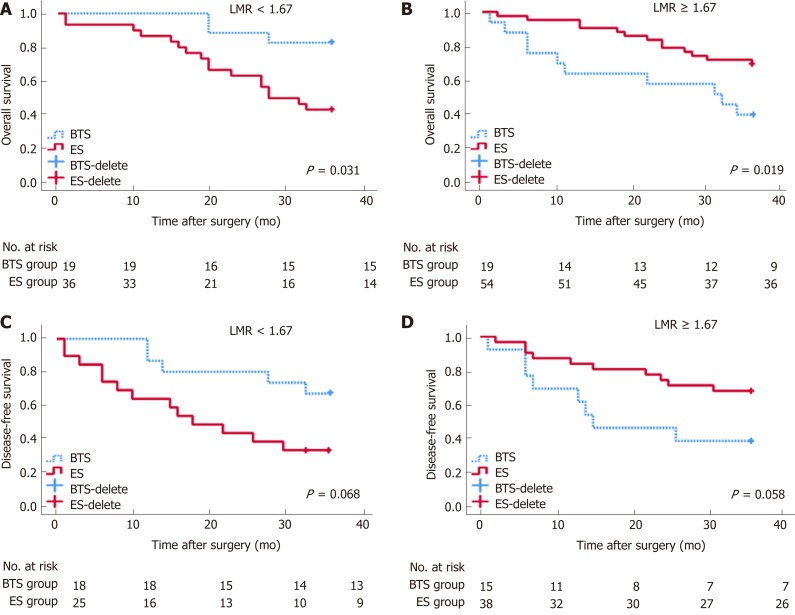
By stratification analysis of 3-year OS and 3-year DFS in different grades of dNLR and LMR, we revealed that only the LMR obviously differentiated the oncological and survival outcomes between the ES and BTS groups. A lower LMR (<1.67), as a protective factor, indicated a lower rate of death (HR = 0.40, 95%CI: 0.18-0.92, P = 0.031) and tumor recurrence (HR = 0.42, 95%CI: 0.17-1.07, P = 0.068) in the BTS group. Conversely, a higher LMR (≥1.67), as a risk factor, showed a higher proportion of death (HR = 4.32, 95%CI: 1.27-14.82, P = 0.019) and tumor recurrence (HR = 2.72, 95%CI: 0.97-7.65, P = 0.058) in the BTS group; these data are presented in Table 7 and Figure 3.
Table 7.
Stratification analysis of oncological and survival outcomes between high-ratio and low-ratio grades in both emergency surgery and bridge to surgery groups
| Characteristic |
3-year OS |
3-year DFS |
||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| ES (dNLR < 1.57) | Reference | - | Reference | - |
| BTS (dNLR < 1.57) | 0.51 (0.18-1.39) | 0.185 | 0.42 (0.13-1.34) | 0.144 |
| ES (dNLR ≥ 1.57) | Reference | - | Reference | - |
| BTS (dNLR ≥ 1.57) | 1.87 (0.79-4.43) | 0.155 | 1.79 (0.77-4.20) | 0.178 |
| ES (LMR < 1.67) | Reference | - | Reference | - |
| BTS (LMR < 1.67) | 4.34 (1.27-14.82) | 0.019 | 2.72 (0.97-7.65) | 0.058 |
| ES (LMR ≥ 1.67) | Reference | - | Reference | - |
| BTS (LMR ≥ 1.67) | 0.40 (0.18-0.92) | 0.031 | 0.42 (0.17-1.07) | 0.068 |
SEMS: Self-expanding metal stents; BTS: Bridge to surgery; dNLR: Derived neutrophil-to-lymphocyte ratio; LMR: Lymphocyte-to-monocyte ratio. P < 0.05 was considered statistically significant.
Figure 3.
Analysis of 3-year overall survival and 3-year disease-free survival, by different lymphocyte-to-monocyte ratios between emergency surgery and bridge to surgery groups. P < 0.05 (log-rank test). Low lymphocyte-to-monocyte ratio (LMR) (LMR < 1.67) indicated higher rates of 3-year OS (A) (HR = 0.40, 95%CI: 0.18-0.92, P = 0.031) and 3-year disease-free survival (DFS) (C) (HR = 0.42, 95%CI: 0.17-1.07, P = 0.068) in the bridge to surgery (BTS) group. Conversely, high LMR (LMR ≥ 1.67) showed lower proportions of 3-year OS (B) (HR = 4.32, 95%CI: 1.27-14.82, P = 0.019) and 3-year DFS (d) (HR = 2.72, 95%CI: 0.97-7.65, P = 0.058) in the BTS group.
DISCUSSION
OCC is always accompanied by a severe local and systemic inflammatory response; some reasons, including the overgrowth of intestinal bacteria, their translocation through the distended colonic wall, and, moreover, septic shock, have been recognized. In this study, we found that the cutoff point for the NLR of 19.30 was much higher than that in elective CRC[14,24], supporting the existing severe systemic inflammation. Although ES and BTS via SEMS insertion have been widely performed, there is still not an objective indication for either. Weighing the balance between oncological outcomes and better preoperative nutritional support with the alleviation of systemic inflammation, BTS via SEMS insertion is only recommended for symptomatic and high surgical risk groups, especially left-side OCC, by the ESGE and World Society of Emergency Surgery (WSES)[1,3]. In this study, analogous with a previous study[25], the BTS group had a higher proportion of LVI (36.80%), though similar 3-year OS and 3-year DFS were observed between the ES and BTS groups. A decreasing tendency in the WBC, NLR, and SII levels was observed after SEMS insertion, which might explain the reason why different inflammation indexes were concluded from the ES (dNLR) and BTS (LMR) groups in our study.
Since 1970, a decreasing peripheral lymphocyte count has been recorded in advanced colon cancer[26], and the inflammation index has been investigated in several kinds of cancer, as it is cost-effective and convenient. The dysbiosis and outgrowth of intestinal microbial species, as a result of acute bowel obstruction and distention, triggers systemic inflammation, leading to the accumulation of neutrophils and monocytes that secrete cytokines and chemokines with the induction of reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI), which might aggravate colonic injury and DNA damage[11]. OCC almost coexists with immunosuppression, which causes a deficiency in adaptive immunologic cells such as T lymphocytes and B lymphocytes, which play important roles in immune surveillance and pathogen depletion[27]. The mechanical stress of SEMS and chronic ablation to the colonic wall enhances local platelet adhesion and the mediation of tumor invasion into lymphovascular vessels[28], which was supported in the current study by a higher proportion of LVI in the BTS group. In this study, we compared different inflammation indexes, including the NLR, dNLR, PLR, LMR and SII, with the CEA level in terms of the predictive value for the prognosis between the ES and BTS groups. Finally, the dNLR was defined as the most efficient index in the ES group; a high dNLR (≥ 1.57) was closely related to low survival benefits, a high incidence of stoma construction, and postoperative pneumonia. Dissimilarly, the LMR was defined as the most efficient index in the BTS group; a high LMR (≥ 1.67) was closely related to low survival benefits and a high incidence of LVI and lymph node invasion.
The reason why different predictive models for the ES and BTS groups were observed in OCC is still unknown. This might be owing to the hypothesis that, as a result of bacterial outgrowth and translocation, OCC always has a severe systemic inflammatory response and immunological deficit, and for patients with a high surgical risk, a BTS via SEMS insertion is preferred. In this study, we found that the BTS group had more severe obstructive symptoms and a bigger tumor size than the ES group. Sufficient alleviation of bowel distention and preoperative nutritional support would improve systemic inflammation and enhance the immunological reaction in the BTS group. However, the mechanical stress of the metal stent might aggravate the local inflammatory response[29-31] and enhance tumor invasion. In our study, with the dramatic decrease of the systemic inflammatory response in the BTS group, the dNLR could not determine the benefit group for ES or BTS. Only the LMR could serve as an objective biomarker for the indication for OCC. A low LMR (< 1.67) was correlated with a low incidence of death and tumor recurrence in the BTS group. Conversely, a high LMR (≥ 1.67) showed a high proportion of death and tumor recurrence in the BTS group, and was preferred for ES.
There were some limitations existing in this study. First, this was a retrospective study in a single center; thus, we will initiate a prospective, multicenter study to confirm our findings. Second, the sample size was not so large that more patients are needed in future research. Furthermore, this study just analyzed the ratio of immune cell populations in the peripheral blood, instead of systematic immune responses including the production of cytokines or expression of PD-1 or CTLA-4. More efforts should be made on the investigation of immune responses occurring in the systemic circulation or tumor.
In conclusion, this study suggests a similar survival and oncological benefits for BTS and ES in patients with OCC. Even though different inflammation indexes for prediction of the prognosis were observed between the ES and BTS groups, they could serve as effective biomarkers. The dNLR was closely related to the prognosis in the ES group, while the LMR was closely related to the prognosis in the BTS group. Specifically, as the potential benefit group, patients with a low LMR might be preferred for BTS via SEMS insertion.
ARTICLE HIGHLIGHTS
Research background
Obstructive colorectal cancer (OCC) presenting with acute abdominal symptoms is always accompanied by severe complications, and the optimal strategy for patients with OCCs remains undetermined. Emergency surgery (ES) and self-expandable metal stents (SEMS) as a bridge to surgery (BTS) were the major treatments for OCCs, however, the indications remain debated. According to different status of immunology and nutrition, predictive factors for prognosis might be different between the two groups. Preoperative inflammation indexes might favor patient selection in terms of the prognosis of OCC.
Research motivation
Weighing the waxes and wanes of ES and BTS, both acute and chronic inflammation responses should be accounted for the selection of optimal patients.
Research objectives
This study was designed to build an inflammatory model for the surgical indications for ES and BTS in OCC.
Research methods
This was a retrospective study in which 128 patients who underwent surgery for OCC at the Department of Emergency Surgery of Fujian Medical University Union Hospital from January 2008 to October 2015 were included in this study. Patients were divided into an ES group and a BTS group according to the surgeon’ advises and patients’ selection. Inflammation indexes were fully evaluated in this study.
Research results
Comparable survival outcomes were observed between the ES and BTS groups. Receiver operating characteristic curve analysis showed dNLR as the optimal biomarker for the prediction of DFS in ES, by contrast, LMR was recommended for BTS with regard to OS and DFS. dNLR was related to stoma construction, postoperative pneumonia, and DFS in the ES group. LMR was closely related to lymph nodes invasion, OS, and DFS in the BTS group. LMR could differentiate OS between the ES and BTS groups. A low LMR (< 1.67) was correlated with a low incidence of death and tumor recurrence in the BTS group.
Research conclusions
As a supplement for the latest ESGE guidelines, the indications for the use of SEMSs in OCC might elaborate to patients with low preoperative LMR, who would benefit from BTS via SEMS insertion.
Footnotes
Institutional review board statement: The study protocol was approved by the Institutional Review Board of Fujian Medical University Union Hospital.
Conflict-of-interest statement: All authors read and approved the final manuscript and declared no conflicts of interest.
Data sharing statement: No additional data are available.
STROBE statement: All the study design and drafting comply with the guidelines of the STROBE statement.
Manuscript source: Unsolicited manuscript
Peer-review started: April 29, 2019
First decision: May 30, 2019
Article in press: July 19, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiba T, Watanabe T S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Xian-Qiang Chen, Department of General Surgery (Emergency Surgery), Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Chao-Rong Xue, Department of General Surgery (Emergency Surgery), Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Ping Hou, Immunotherapy Institute, Fujian Medical University, Fuzhou 350122, Fujian Province, China.
Bing-Qiang Lin, Department of General Surgery (Emergency Surgery), Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China.
Jun-Rong Zhang, Department of General Surgery (Emergency Surgery), Fujian Medical University Union Hospital, Fuzhou 350001, Fujian Province, China. junrongzhang@fjmu.edu.cn.
References
- 1.van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A European Society of Gastrointestinal Endoscopy (ESGE) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc. 2014;80:747–61.e1-75. doi: 10.1016/j.gie.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Yeo HL, Lee SW. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg. 2013;17:2007–2012. doi: 10.1007/s11605-013-2343-x. [DOI] [PubMed] [Google Scholar]
- 3.Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. doi: 10.1186/s13017-018-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindarajan A, Naimark D, Coburn NG, Smith AJ, Law CH. Use of colonic stents in emergent malignant left colonic obstruction: a Markov chain Monte Carlo decision analysis. Dis Colon Rectum. 2007;50:1811–1824. doi: 10.1007/s10350-007-9047-9. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballón P, Moreno-Azcoita M. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401–406. doi: 10.1007/s10350-004-6190-4. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi G, Yamada T, Iwai T, Takeda K, Koizumi M, Shinji S, Uchida E. Oncological Assessment of Stent Placement for Obstructive Colorectal Cancer from Circulating Cell-Free DNA and Circulating Tumor DNA Dynamics. Ann Surg Oncol. 2018;25:737–744. doi: 10.1245/s10434-017-6300-x. [DOI] [PubMed] [Google Scholar]
- 7.Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA collaborative Dutch Stent-In study group. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg. 2014;101:1751–1757. doi: 10.1002/bjs.9645. [DOI] [PubMed] [Google Scholar]
- 8.Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD Association of Coloproctology of Great Britain, Ireland. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76–81. doi: 10.1097/01.sla.0000130723.81866.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraguchi N, Ikeda M, Miyake M, Yamada T, Sakakibara Y, Mita E, Doki Y, Mori M, Sekimoto M. Colonic stenting as a bridge to surgery for obstructive colorectal cancer: advantages and disadvantages. Surg Today. 2016;46:1310–1317. doi: 10.1007/s00595-016-1333-5. [DOI] [PubMed] [Google Scholar]
- 10.Palin RP, Devine AT, Hicks G, Burke D. Association of pretreatment neutrophil-lymphocyte ratio and outcome in emergency colorectal cancer care. Ann R Coll Surg Engl. 2018;100:308–315. doi: 10.1308/rcsann.2017.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buettner S, Spolverato G, Kimbrough CW, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Weiss M, Bauer TW, Shen F, Poultsides GA, Marsh JW, IJzermans JNM, Koerkamp BG, Pawlik TM. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery. 2018;164:411–418. doi: 10.1016/j.surg.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Farolfi A, Petrone M, Scarpi E, Gallà V, Greco F, Casanova C, Longo L, Cormio G, Orditura M, Bologna A, Zavallone L, Ventriglia J, Franzese E, Loizzi V, Giardina D, Pigozzi E, Cioffi R, Pignata S, Giorda G, De Giorgi U. Inflammatory Indexes as Prognostic and Predictive Factors in Ovarian Cancer Treated with Chemotherapy Alone or Together with Bevacizumab. A Multicenter, Retrospective Analysis by the MITO Group (MITO 24) Target Oncol. 2018;13:469–479. doi: 10.1007/s11523-018-0574-1. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Ding L, Yang GG, Qiu JM, Wang D, Wang H, Fu C. Predictive impact of the inflammation-based indices in colorectal cancer patients with adjuvant chemotherapy. Cancer Med. 2018 doi: 10.1002/cam4.1542. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JX, Li A, Zhou LY, Liu XF, Wei ZH, Wang XZ, Ying HQ. Significance of combined preoperative serum Alb and dNLR for diagnosis of pancreatic cancer. Future Oncol. 2018;14:229–239. doi: 10.2217/fon-2017-0339. [DOI] [PubMed] [Google Scholar]
- 16.Diakos CI, Tu D, Gebski V, Yip S, Wilson K, Karapetis CS, O'Callaghan CJ, Shapiro J, Tebbutt N, Jonker DJ, Siu LL, Wong R, Doyle C, Strickland AH, Price TJ, Simes J, Clarke S. Is the derived neutrophil to lymphocyte ratio (dNLR) an independent prognostic marker in patients with metastatic colorectal cancer (mCRC)? Analysis of the CO.17 and CO.20 studies. Ann Oncol. 2016;27:588P. [Google Scholar]
- 17.Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017;265:539–546. doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 19.Lin BQ, Wang RL, Li QX, Chen W, Huang ZY. Investigation of treatment methods in obstructive colorectal cancer. J BUON. 2015;20:756–761. [PubMed] [Google Scholar]
- 20.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 21.Wittekind C. [2010 TNM system: on the 7th edition of TNM classification of malignant tumors] Pathologe. 2010;31:331–332. doi: 10.1007/s00292-010-1349-3. [DOI] [PubMed] [Google Scholar]
- 22.Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I, Shimada M, Yamada T, Tomita M, Koizumi K, Hirata N, Kanazawa H, Enomoto T, Sekido H, Saida Y. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016;30:3976–3986. doi: 10.1007/s00464-015-4709-5. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–7; discussion 937-9. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017;115:470–479. doi: 10.1002/jso.24523. [DOI] [PubMed] [Google Scholar]
- 26.Sabbagh C, Chatelain D, Trouillet N, Mauvais F, Bendjaballah S, Browet F, Regimbeau JM. Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc. 2013;27:3622–3631. doi: 10.1007/s00464-013-2934-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim US, Papatestas AE. Letter: Peripheral lymphocyte counts in colonic disease. Lancet. 1974;2:462–463. doi: 10.1016/s0140-6736(74)91843-1. [DOI] [PubMed] [Google Scholar]
- 28.Menges T, Engel J, Welters I, Wagner RM, Little S, Ruwoldt R, Wollbrueck M, Hempelmann G. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27:733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A. 2012;109:911–916. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BG, Gao MQ, Kang S, Choi YP, Lee JH, Kim JE, Han HH, Mun SG, Cho NH. Mechanical compression induces VEGFA overexpression in breast cancer via DNMT3A-dependent miR-9 downregulation. Cell Death Dis. 2017;8:e2646. doi: 10.1038/cddis.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]