Figure 7.
An Asymmetric Hand-over-Hand Rotational Mechanism for CMG Translocation
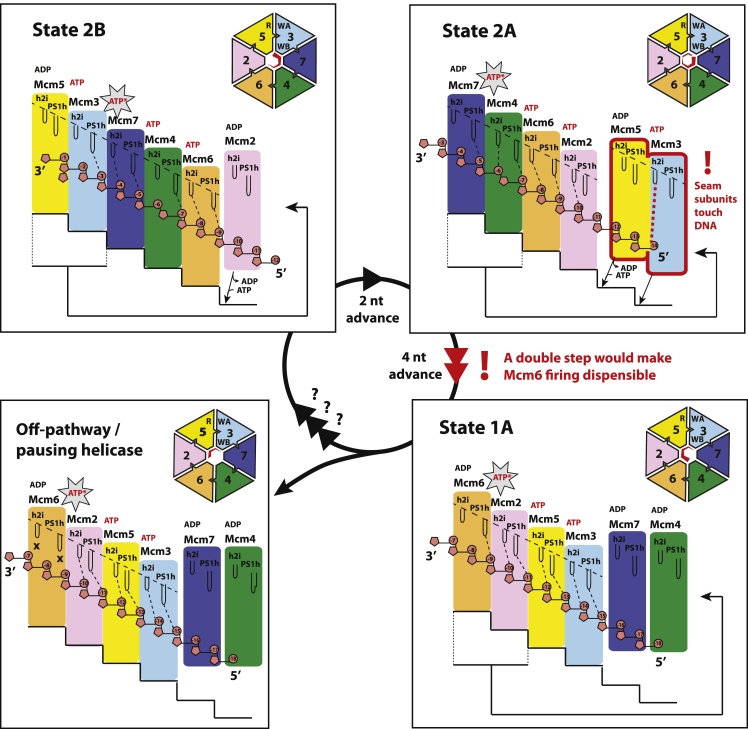
Transition from states 2B to 2A involves release of 3′ DNA by Mcm3 PS1h and ATP binding by Mcm2, which binds to the N-terminal end of the AAA+ staircase. At the same time, Mcm3 h2i pore loop touches the incoming 5′ DNA on the N-terminal side of MCM. In state 2A, seam subunits Mcm5 and Mcm3 sandwich ATP. ATP-Mcm5 association with the N-terminal side of the AAA+ staircase would drag ATP-Mcm3 along, causing detachment of two subunits from the C-terminal end of the spiral. As a result, Mcm6 is found at the C-terminal end of the AAA+ staircase (state 1A). As Mcm5-3 binding to the ATPase spiral may occur en bloc, this would render ATP hydrolysis by Mcm6 dispensable. We speculate that state 1B, where Mcm6 is disengaged from DNA, might be off pathway and represent a pausing state. Alternatively, it might represent a corrupted version of state 1A, which partially disassembled from DNA during handling or freezing. Star marks ATPase-competent site.