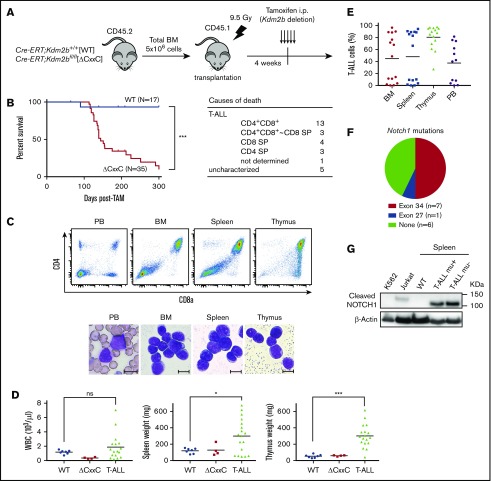
Figure 2.
ΔCxxC mice developed T-ALL. (A) Experimental design of the hematopoietic-specific deletion of Kdm2b exon 13. (B) Kaplan-Meier survival curves of WT (n = 17) and ΔCxxC (n = 35) mice after the injection of tamoxifen. Data from 4 independent experiments were combined. P values were calculated by the log-rank test. The causes of death in ΔCxxC mice are summarized in the table. (C) Representative flow cytometric profiles and smear preparation of PB and cytospin preparations of BM and the thymus after May-Giemsa staining. Hematopoietic cells were obtained from the PB, BM, spleen, and thymus of a DP-type T-ALL mouse. Scale bars, 10 μm. (D) PB WBC counts and spleen and thymus weights in moribund ΔCxxC T-ALL mice (n = 18) and WT (n = 7) and ΔCxxC (n = 4) mice 300 days after the injection of tamoxifen. Bars in scatter diagrams indicate median values. (E) Proportions of T-ALL cells in BM, the spleen, thymus, and PB. Bars in scatter diagrams indicate median values. (F) Frequencies of Notch1 active mutations in exons 27 and 34 detected in T-ALL cells sorted from thymus. (G) Cleaved NOTCH1 protein in T-ALL cells detected by a western blot analysis. Cleaved NOTCH1 proteins in human K562 cells (negative control), Jurkat T-ALL cells (positive control), and spleen cells from WT and ΔCxxC T-ALL mice with and without Notch1 mutations (mu+/−) are shown. Actin served as a loading control. *P < .05; ***P < .001 by Student t test.