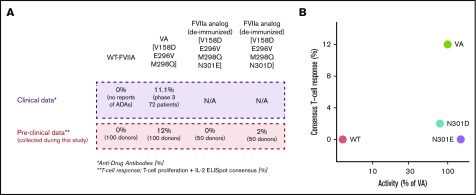
Figure 6.
Molecules with desired functional activity and lower immunogenicity risk. (A) Correlation of preclinical data with clinical outcomes. (B) The relation between activity and T-cell response in all evaluated FVII variants. N301E and N301D exhibit desired characteristics for a therapeutic-protein (ie, low T-cell response and activity comparable to the VA). T-cell responses were measured using 2 independent experiments of 50 donors each. In experiment 1, PBMCs from 50 donors were exposed to WT-rFVII and VA sequences. In experiment 2, PBMCs from 50 donors were exposed to WT-rFVIIa, VA, and deimmunized sequences (N301E and N301D). Activities for each FVII variant are represented as a mean calculated from 2 independent protein preparations using VA as a standard (100%).