Figure 1.
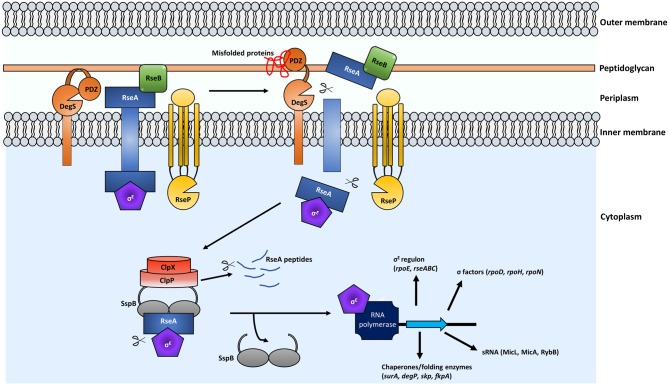
Regulated intramembrane proteolysis (RIP) leads to activation of the σE ESR. In the presence of misfolded proteins, a conformational change as a result of misfolded proteins binding to the PDZ domain of DegS occurs. Subsequently the protease domain of DegS is exposed and the periplasmic domain of RseA is cleaved. RseP then cleaves RseA at the cytoplasmic domain, releasing the σE-bound RseA portion into the cytoplasm. Binding of the adaptor protein SspB to RseA-σE recruits the ClpXP protease for degradation of RseA and release of free σE. σE binds to RNA polymerase and transcription of the σE regulon is induced.