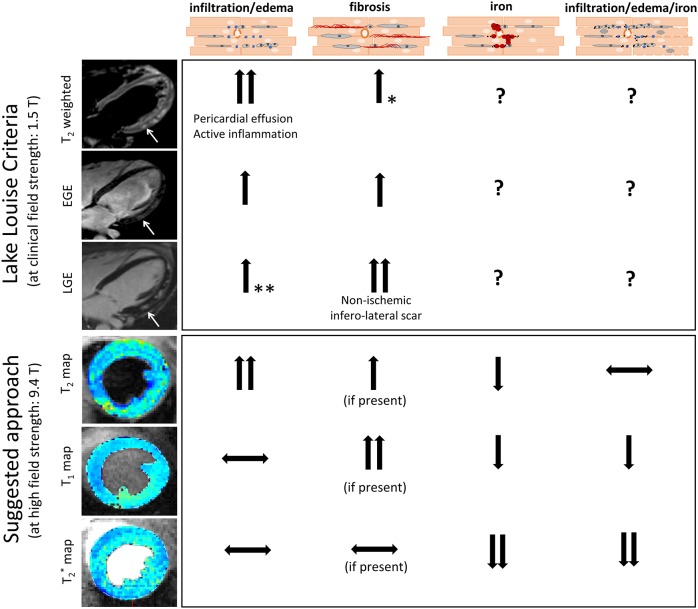
Fig. 5.
Comparison of the CMR protocol presented in this study and the established diagnostic approach for myocarditis in human patients based on the Lake Louise criteria (LLC). LLC-based measurements in human patients using clinical MRI field strengths. Edema leads to increased T2 weighted signal and increased T2 relaxation times, suggestive of acute inflammation and/or pericardial effusion. Hyperemia leads to increased early gadolinium enhancement (EGE) caused by leakage of gadolinium from capillaries. Necrosis or fibrosis can be identified by late gadolinium enhancement (LGE) because gadolinium crosses the damaged myocyte membrane (necrosis) or accumulates in the extended extracellular space (fibrosis). Example CMR images illustrate an example of increased edema (increased T2), capillary leakage (increased EGE) and nonischemic scar (positive LGE) at the inferolateral wall of a human myocarditis patient. *Fibrosis can also nonspecifically affect T2 values; however, T2 alone is not sufficient to diagnose fibrosis but needs to be combined with T1 or LGE measurements. **LGE can be enhanced in the presence of edema, but should not be used as a marker to identify myocardial edema, which should be done using T2 mapping. The approach proposed in this study and optimized on Resiquimod-treated mice with hemorrhagic myocarditis provides a contrast agent-free CMR protocol able to detect inflammatory damage and allows correct interpretation of pathology, despite potential interference from iron with the traditional T1 and T2 measures. In this model, edema increased and iron decreased T2 relaxation times, and values appeared unchanged in the presence of both edema and iron due to their opposing effects. Native T1 did not change in the presence of edema, and decreased in the presence of iron. T1 is expected to increase in the presence of fibrosis. T2* strongly decreases in the presence of iron and is not expected to change significantly in response to edema or fibrosis.