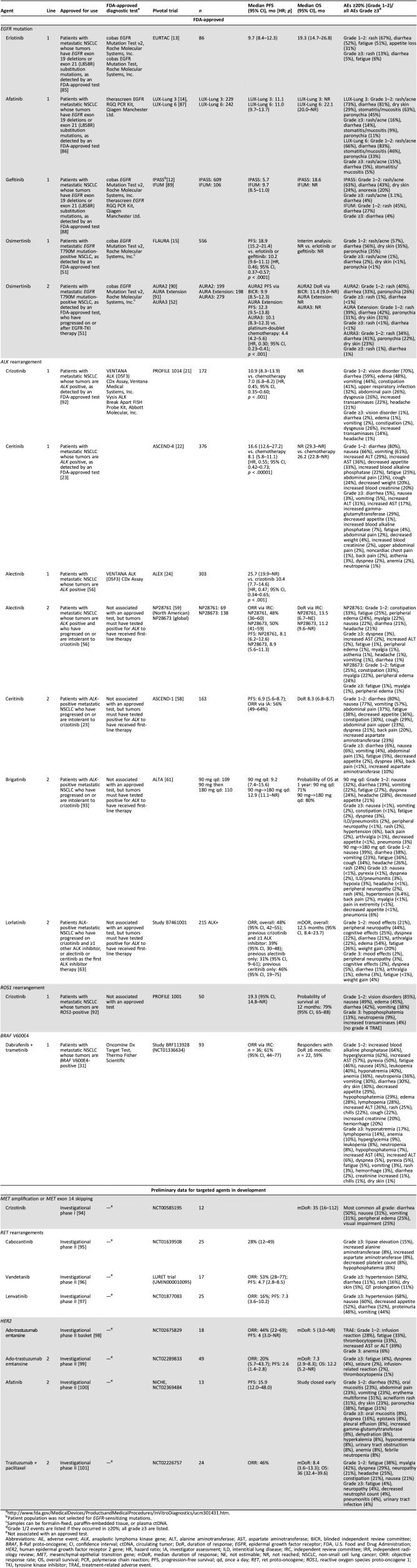
Table 1. Summary of data on efficacy and safety of targeted agents in metastatic NSCLC.
Patient population was not selected for EGFR‐sensitizing mutations.
Samples can be formalin‐fixed, paraffin‐embedded tissue, or plasma ctDNA.
Grade 1/2 events are listed if they occurred in ≥20%; all grade ≥3 are listed.
Not associated with an approved test.
Abbreviations: AE, adverse event; ALK, anaplastic lymphoma kinase gene; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BICR, blinded independent review committee; BRAF, B‐Raf proto‐oncogene; CI, confidence interval; ctDNA, circulating tumor; DoR, duration of response; EGFR, epidermal growth factor receptor; FDA, U.S. Food and Drug Administration; HER2, human epidermal growth factor receptor 2 gene; HR, hazard ratio; IA, investigator assessment; ILD, interstitial lung disease; IRC, independent review committee; IRR, independent radiology review; MET, mesenchymal‐epithelial transition gene; mDoR, median duration of response; NE, not estimable; NR, not reached; NSCLC, non‐small cell lung cancer; ORR: objective response rate; OS, overall survival; PCR, polymerase chain reaction; PFS, progression‐free survival; qd, once a day; RET, ret proto‐oncogene; ROS1, reactive oxygen species proto‐oncogene 1; TKI, tyrosine kinase inhibitor; TRAE, treatment‐related adverse event.