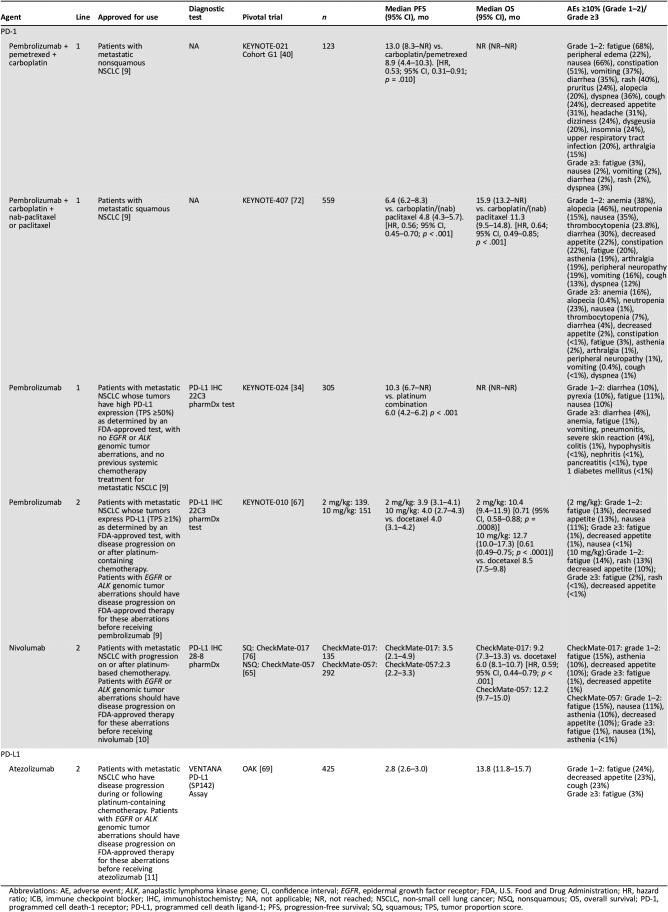
Table 2. Summary of data on efficacy and safety of ICBs approved for use in metastatic NSCLC.
Abbreviations: AE, adverse event; ALK, anaplastic lymphoma kinase gene; CI, confidence interval; EGFR, epidermal growth factor receptor; FDA, U.S. Food and Drug Administration; HR, hazard ratio; ICB, immune checkpoint blocker; IHC, immunohistochemistry; NA, not applicable; NR, not reached; NSCLC, non‐small cell lung cancer; NSQ, nonsquamous; OS, overall survival; PD‐1, programmed cell death‐1 receptor; PD‐L1, programmed cell death ligand‐1; PFS, progression‐free survival; SQ, squamous; TPS, tumor proportion score.