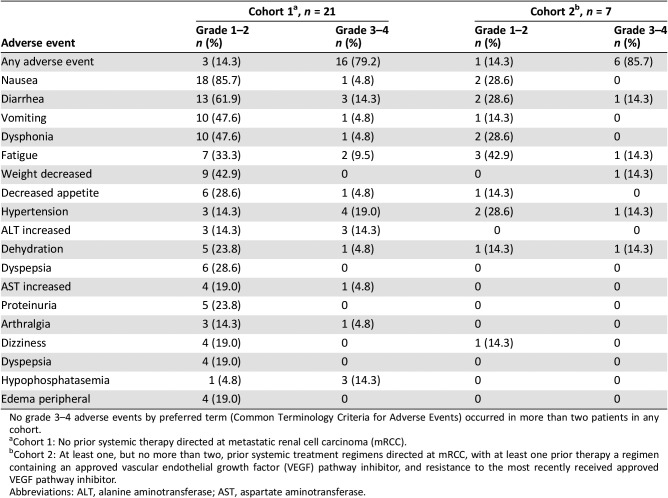
Table 5. Adverse events by Common Terminology Criteria for Adverse Events grade reported in more than three patients during the dose‐expansion phase (patients with metastatic renal cell carcinoma).
No grade 3–4 adverse events by preferred term (Common Terminology Criteria for Adverse Events) occurred in more than two patients in any cohort.
Cohort 1: No prior systemic therapy directed at metastatic renal cell carcinoma (mRCC).
Cohort 2: At least one, but no more than two, prior systemic treatment regimens directed at mRCC, with at least one prior therapy a regimen containing an approved vascular endothelial growth factor (VEGF) pathway inhibitor, and resistance to the most recently received approved VEGF pathway inhibitor.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.