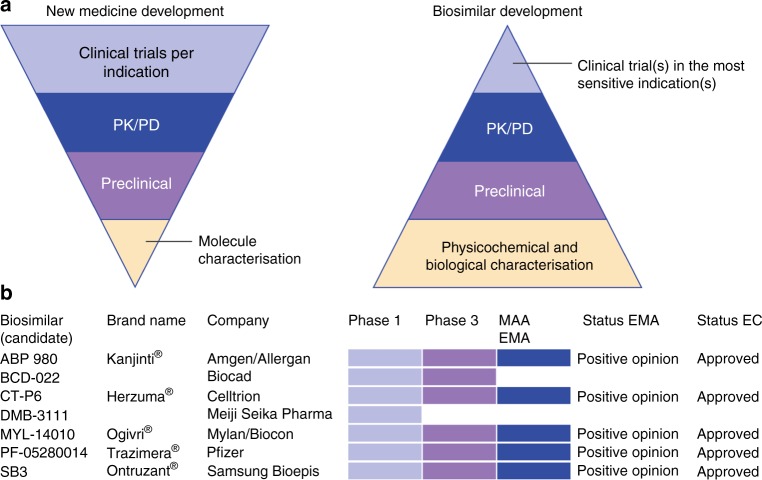
Fig. 1.
Biosimilar development: an overview of the development pathway and the different trastuzumab biosimilar(s) (candidates) approved or in clinical development. a New medicine versus biosimilar medicine development. Adapted from McCamish (2011) Mabs.93 b Key trastuzumab biosimilar candidates approved or in clinical development (status December 2018). EC: European Commission, EMA: European Medicines Agency, MAA: marketing authorisation application