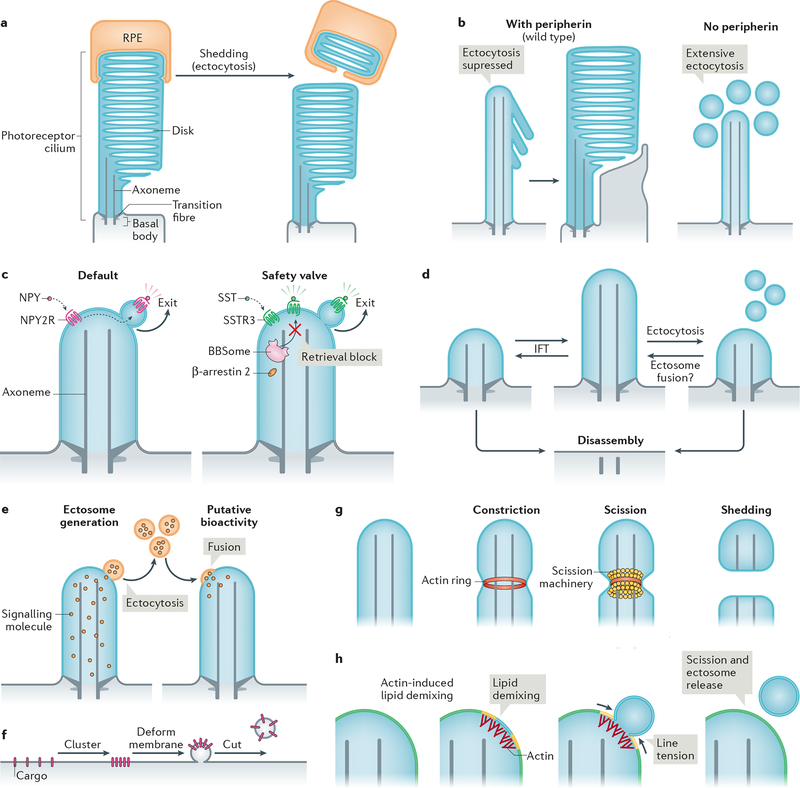
Figure 4|. Modalities and mechanisms of ciliary ectocytosis.
a| The tips of photoreceptor outer segments are shed daily to remove aged rhodopsin molecules. Shed material is phagocytosed by retinal pigmented epithelial (RPE) cells (and recycled back to photoreceptors; not shown) (left). Ectocytosis also negatively regulates outer segment morphogenesis: the presence of disk protein peripherin blocks ectocytosis and enables retention of ciliary components to form disks (right). b| Upon activation, some G protein-coupled receptors (GPCRs) are removed from cilia by ectocytosis. For example, neuropeptide Y receptor type 2 (NPY2R) uses ectocytosis as a primary mechanism for cilia exit (left). Activated somatostatin receptor 3 (SSTR3) becomes ectocytosed when its BBSome-based retrieval (see Fig. 3b) is blocked (right). NPY, neuropeptide Y, SST, somatostatin. c| Intraflagellar transport (IFT) elongates or shrinks the cilium by exchanging material with the rest of the cell. Ectocytosis may serve as an alternative mechanism of ciliary length homeostasis: shedding of fragments of cilia from the tip by ectocytosis reduces ciliary length, whereas the hypothetical fusion of extracellular vesicles with the cilium may increase cilia length. d| Cilia package signalling molecules into ectosomes. These may be received by other cells to confer putative bioactivity. As exemplified in Chlamydomonas reinhardtii, recipient cells may receive extracellular vesicles via their cilia; incorporation via the plasma membrane is also conceivable. e| Basic steps of extracellular vesicle formation: clustering of cargoes is followed by membrane deformation and finally, a forming vesicle is released from the donor membrane by scission. f| Actin may serve to constrict the diameter of the cilium by forming a contractile ring prior to scission driven by an additional scission machinery, such as ESCRT-III-based complexes. g| Model of how actin may function in ectosome budding and scission. For simplicity, the ciliary membrane is depicted as consisting of two lipid species (yellow and blue, which blend to green). Actin polymerization in cilia causes local lipid demixing. Line tension at the interface between lipid phases ensues, which is resolved by budding and scission of the de-mixed lipids, releasing a vesicle.