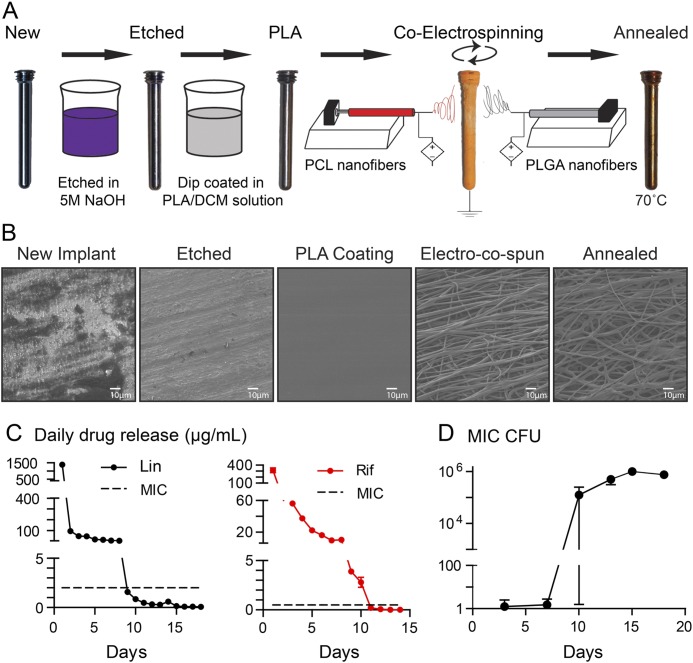
Fig. 1.
Figs. 1-A through 1-D Antibiotic-loaded implant coating. Fig. 1-A The nanofiber antibiotic-releasing coating was prepared using base-etched orthopaedic-grade titanium peg implants, which were then dip-coated in PLA dissolved in dichloromethane (PLA/DCM) solution. Co-electrospinning was performed to coat the implants with PLGA and PCL nanofibers loaded with or without antibiotics followed by heat treatment (annealing) to generate a conformal PCL and PLGA fiber coating. Fig. 1-B Representative scanning electron microscopy images of the implant and nanofiber coating surfaces after each step in the coating process. Fig. 1-C In vitro antibiotic release of linezolid (Lin) or rifampin (Rif) (mean µg/mL and SEM) measured by placing the coated implants into a new solution of PBS daily for 18 days (n = 6 coated implants per group). The horizontal dotted lines show the minimum inhibitory concentration (MIC) of SAP231 for Lin (2 μg/mL) and Rif (0.5 μg/mL). Fig. 1-D In vitro antimicrobial activity assays were performed by incubating the drug-release media from the Lin/Lin + Rif-coated implants collected daily and analyzed on days 3, 7, 10, 13, 15, and 18. CFUs (mean and SEM, logarithmic scale) were enumerated by absorbance (A600) and a CFU standard curve for the coatings (n = 6 coated implants per group).