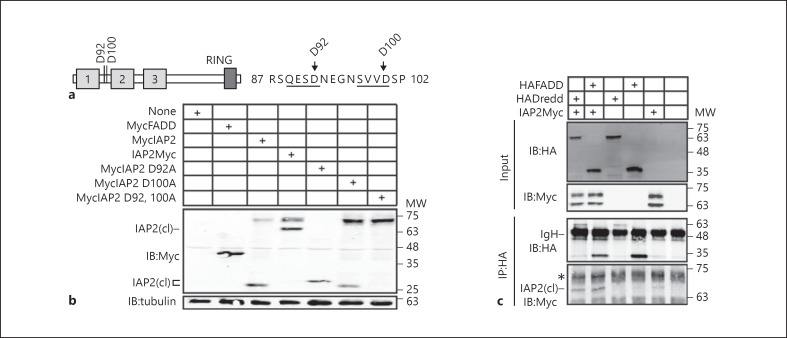
Fig. 6.
a Schematic illustration of IAP2 with putative caspase cleavage sites at residues 92 and 100. The primary amino acid sequences of residues 87–102 are also shown with putative caspase recognition sites underlined. b Western blot analysis of lysates from S2 cells transfected with the expression constructs indicated in the tables at the top of each gel. Total cell lysates were immunoblotted with anti-myc antibodies where indicated, and tubulin levels were visualized as a loading control. N-terminal IAP2 cleavage products are indicated with IAP2(cl). c Co-immunoprecipitation studies of lysates from S2 cells that were transfected with the expression constructs indicated at the top of the gel, and immunoprecipitated with a monoclonal anti-HA antibody. Input and immunoprecipitate fractions were visualized with anti-HA and anti-myc antibodies as indicated. MW is shown for each blot in kilodaltons.