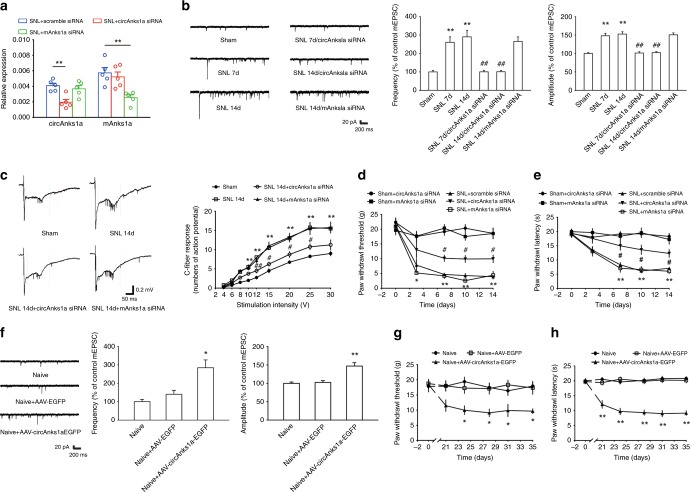
Fig. 3.
Upregulation of circAnks1a-mediated neuropathic pain following SNL. a Application of circAnks1a siRNA but not mAnks1a siRNA (1 nmol 10 µl−1 i.t. for consecutive 5 days) knocked down the expression of circAnks1a on day 14 following SNL (**P < 0.01 vs. the corresponding scramble group, two-tailed one-way ANOVA, n = 5). b CircAnks1a siRNA treatment ameliorated the increase in the amplitude and frequency of mEPSCs on days 7 and 14 induced by SNL (**P < 0.01 vs. the sham group, ##P < 0.01 vs. the corresponding SNL group, two-tailed one-way ANOVA for amplitude, two-tailed permutation tests for frequency, n = 22). c The number of C-fiber–evoked action potentials increased following SNL (**P < 0.01 vs. the sham group, #P < 0.05, ##P < 0.01 vs. the corresponding SNL group, two-tailed one-way ANOVA, n = 4). d Intrathecal injection of circAnks1a siRNA attenuated the mechanical allodynia induced by SNL (*P < 0.05, **P < 0.01 vs. the sham + circAnks1a siRNA group, #P < 0.05 vs. the SNL + scramble siRNA group, two-tailed permutation tests, n = 10). e The effect of circAnks1a siRNA on withdrawal latency (**P < 0.01 vs. the sham + circAnks1a siRNA group, #P < 0.05 vs. the SNL + scramble siRNA group, two-tailed permutation tests, n = 12). f Intraspinal injection of recombinant AAV-circAnks1a-EGFP increased the amplitude and frequency of mEPSCs (*P < 0.05, **P < 0.01 vs. the AAV-EGFP group, two-tailed one-way ANOVA for amplitude, two-tailed permutation tests for frequency, n = 18). g Recombinant AAV-circAnks1a-EGFP significantly reduced the withdrawal threshold 21 days after injection (*P < 0.05 vs. the AAV-EGFP group, two-tailed permutation tests, n = 12). h The effect of AAV-circAnks1a-EGFP on withdrawal latency (**P < 0.01 vs. the AAV-EGFP, two-tailed permutation tests, n = 12). The data are presented as the mean ± s.e.m. Source data are available as a Source Data file