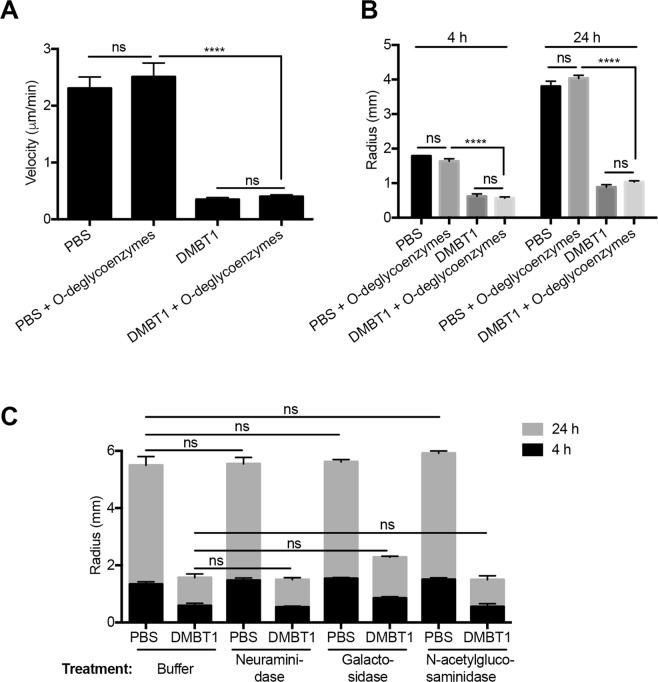
Figure 7.
O-Glycosylation of DMBT1 does not contribute to inhibition of bacterial twitching motility. (A) Effect of DMBT1 digested by an O-deglycosylation enzyme mix on twitching velocity of P. aeruginosa PAO1 after 4 h. DMBT1 (100 ng/µL) was digested by a mixture of O-deglycosylation enzymes (O-glycosidase, Neuraminidase, Galactosidase and N-acetyl-glucosaminidase) at 37 °C for 2 days in native glycobuffer (50 mM sodium phosphate, pH 7.5) to remove complex O-glycosylation of DMBT1. PBS and DMBT1 diluted with same volume of glycobuffer served as controls. (B) Effects of DMBT1 digested by the O-deglycosylation enzyme mix on colony size of P. aeruginosa PAO1 after 4 h and 24 h. (C) Effects of differently digested DMBT1 on colony size of P. aeruginosa PAO1 after 4 h and 24 h. DMBT1 (100 ng/µL) was digested by Neuraminidase (desialylation glycosidase), Galactosidase or N-acetylglucosaminidase (both exoglycosidases) at 37 °C for 2 h in glycobuffer (50 mM sodium acetate, 0.5 mM CaCl2, pH 5.5) to remove sialic acid residues and other complex glycosylation of DMBT1. PBS and DMBT1 diluted with same volume of glycobuffer served as controls. Data shown as the mean ± SEM per sample from three independent experiments. Significance was determined using one-way ANOVA (panel A) or two-way ANOVA (panel B) each with Tukey’s multiple comparisons, ****P < 0.0001; ns, not significant.