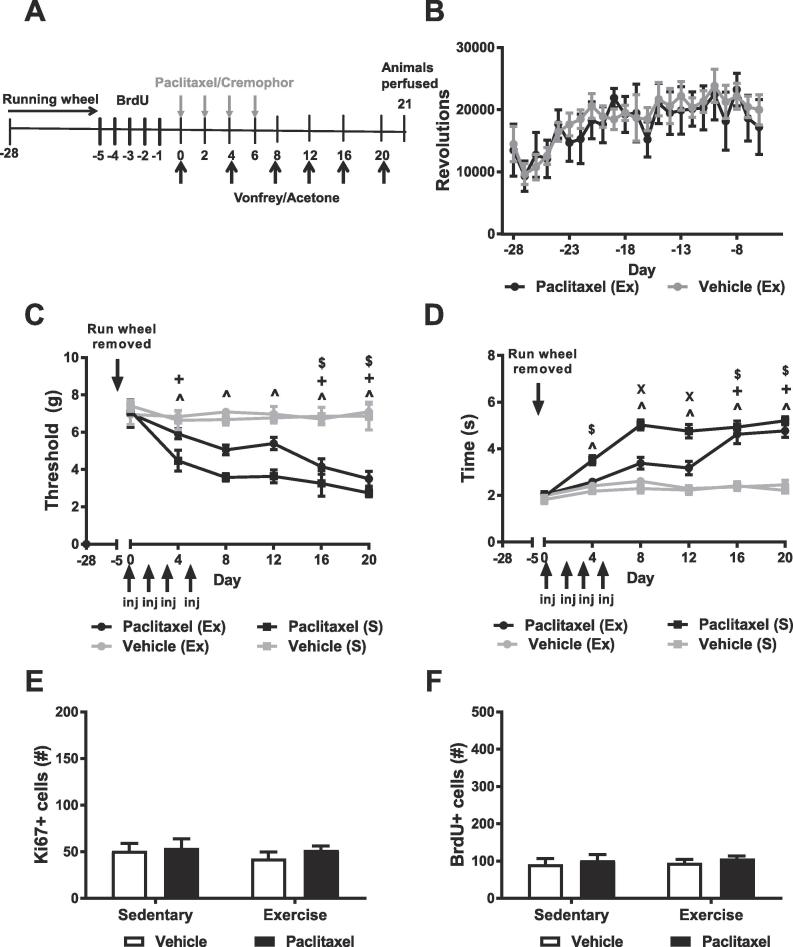
Fig. 2.
Voluntary running terminated prior to CIPN partially alleviates paclitaxel-induced allodynia. (A) Schematic shows timing of experimental procedures in Study 2. Voluntary running was initiated 28 days prior to paclitaxel or vehicle injections. Paclitaxel (4 mg/kg i.p.) or vehicle was administered on day 0, 2, 4 and 6. Animals were tested for mechanical and cold hypersensitivity for a period of 20 days followed by perfusion on day 21 post paclitaxel or vehicle injection. BrdU (100 mg/kg i.p.) was administered once daily across 5 consecutive days prior to initiation of dosing with paclitaxel or its vehicle following termination of access to running wheels or no wheels. (B) Running rates did not differ between groups prior to initiation of treatment with paclitaxel or vehicle. Prior voluntary wheel running delayed development of paclitaxel-induced (C) mechanical and (D) cold hypersensitivities. This anti-allodynic effect persisted up to 16 days following the initiation of paclitaxel dosing. The overall number of Ki67 (E) or BrdU (F) expressing cells did not differ between groups irrespective of chemotherapy or exercise condition. Voluntary running terminated (i.e. mice were given free access to running wheels and had running wheels removed 23 days later) prior to the start of paclitaxel/vehicle treatment. Data are expressed as mean ± SEM. N = 5–6 per group. ^p < 0.05 Paclitaxel Sedentary vs. Vehicle Exercise/Sedentary, +p < 0.05 Paclitaxel Exercise vs. Vehicle Exercise/Sedentary, $p < 0.05 paclitaxel Exercise vs. vehicle, Xp < 0.05 Paclitaxel Exercise vs. Paclitaxel Sedentary two-way ANOVA followed by Bonferroni post-hoc test. Exercise, Ex, indicates subjects exposed to running wheels. Sedentary, S, indicates subjects housed without running wheels. Arrows denoting injections (inj) denote timing of paclitaxel or vehicle injection. Horizontal arrow denotes timing of voluntary running.