Figure 1.
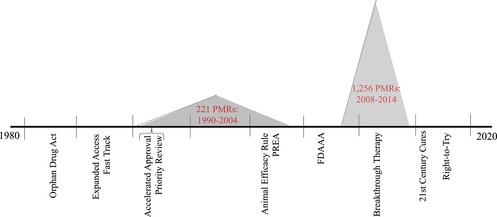
Timeline of Key Policy Changes, Adoption of Expedited Programs, and Increasing Use of Postmarket Requirementsa
aThe PMR data incorporated in this figure derive from two reports prepared by the US Department of Health and Human Services’ Office of Inspector General.5, 85 The two reports present PMR data differently; in particular, the 2006 report simply presents a lump sum total of all PMRs issued by FDA during the years 1990‐2004, as opposed to the number of PMRs issued per year, which the 2016 report documents. For consistency, a lump sum total of PMRs is therefore presented for the period covered by the 2016 report, ie, 2008‐2014. Importantly, the main contributor to the growth of PMRs in the latter period of time is the addition of the authority to issue PMRs under FDAAA, which was enacted in 2007 and came into force in 2008. As explained by OIG,85 (p8) the “number of PMRs that FDA issued increased by 111% from FY 2008 to FY 2009, and then remained fairly consistent through FY 2014.” [Color figure can be viewed at http://wileyonlinelibrary.com]
