Introduction
In Canada, the incidence and prevalence of heart failure (HF) is rising. Approximately 600,000 patients are living with HF and 50,000 more are diagnosed each year. The burden of HF is significant. Mortality rate is high (20% in 1 year, 50% in 5 years), and morbidity, such as hospitalizations, is frequent. The cost to the health care system is significant at $2.8 billion per year.1
In 2017, the Canadian Cardiovascular Society published a comprehensive update to the heart failure management guidelines.2 These guidelines aimed to provide broad recommendations across the spectrum of HF. The full document can be viewed at www.onlinecjc.ca/article/S0828-282X(17)30973-X/pdf.
In this summary of the guidelines, we aim to highlight the recommendations that are most relevant to the practising pharmacist, with a focus on chronic HF with reduced ejection fraction since this subgroup of HF has the most evidence for pharmacotherapy.
Pharmacist roles in heart failure
Heart failure is a complex disease, requiring polypharmacy and often involving multiple comorbidities. Due to these complexities and the fact that pharmacists are the most easily accessible and frequently seen health care provider, pharmacists can play a significant role in the management of HF. There is a large body of evidence supporting the role of the pharmacist specifically in caring for patients with HF. The following activities can be performed by pharmacists in multiple settings when caring for patients with HF.
Medication reconciliation/medication reviews
Patients with HF are on multiple medications and changes are made frequently; therefore, medication reconciliation is very important, especially at transfers of care and discharges back to community. Medication review and reconciliation can increase adherence, reduce readmissions and reduce cost to the system.3,4
Promotion of medication adherence
Being aware of factors that could influence adherence, such as being a visible minority, living alone, having multiple comorbidities and/or depression,5 can help identify patients who may require more interventions from the pharmacist. Interventions include clarifying instructions on labels, intensifying education and closer follow-up.
Discharge counselling and education after discharge have also been shown to improve adherence in patients with HF.6-8
Education
Pharmacists should educate their patients on medications, management of side effects, self-monitoring, symptom management, dietary modifications and lifestyle modifications in HF. Many of these points will be covered in this summary of the heart failure guidelines.
Pharmacist education of HF patients has been shown to reduce readmissions for heart failure, decrease length of stay in hospital,9 improve survival10 and improve symptoms.11
Medication management
Heart failure treatment requires multiple medications with a large potential for interactions, side effects and changes. Pharmacists are perfectly positioned to help with the initiation, titration and monitoring of HF therapies. Pharmacist involvement has been shown to reduce medication errors, adverse drug reactions and costs to the health care system.12-16
Drug interactions: Pharmacists can identify and prevent adverse drug reactions and interactions. For example, they can identify improper use of medications that could worsen HF such as nonsteroidal anti-inflammatory drugs (NSAIDs). They are also a great resource for patients who are using complementary and alternative medicines to check for drug and disease interactions or contraindications.
Therapy optimization: Studies have shown that HF medications can be underused or underdosed. Pharmacists can help to identify and intervene for those patients who require further optimization of their medications.17 Such interventions, when made by pharmacists, have been shown to improve survival and improve HF symptoms.18,19
Transitions in care: After discharge, many HF patients require close follow-up, and pharmacists can be a resource before they have their next follow-up appointment. Pharmacist-led bridge clinics postdischarge have been found to decrease mortality in HF patients.20
Advocacy and public health
Vaccination against influenza and pneumococcal disease of these high-risk patients is also a key role that pharmacists can play in caring for these patients.
Definition of heart failure
Heart failure is a complex clinical syndrome in which abnormal heart function results in, or increases the subsequent risk of, clinical signs and symptoms of reduced cardiac output and/or pulmonary or systemic congestion at rest or with stress. Chronic HF refers to the persistent and progressive state of HF, and acute HF is a gradual or rapid change in signs and symptoms.
Historically, left ventricular systolic dysfunction has been the major focus, but the syndrome of HF can result from a variety of conditions relating to both the structure (e.g., left or right sided, valvular, etc.) and function of the heart. New terminology has arisen to help describe different HF manifestations based on left ventricular (LV) function (Table 1). This terminology helps determine the approach to medical management for these patients.
Table 1.
Heart failure (HF) terminology and corresponding left ventricular ejection fraction
| Terminology | Left ventricular ejection fraction, % |
|---|---|
| HF with preserved ejection fraction (HFpEF) | ≥50 |
| HF with midrange ejection fraction (HFmEF) | 41-49 |
| HF with reduced ejection fraction (HFrEF) | ≤40 |
Those with an ejection fraction (EF) that is <40% with current or previous signs and symptoms of heart failure are referred to as having heart failure with reduced ejection fraction (HFrEF). This was previously termed systolic dysfunction. It should also be noted that patients may have an improvement or normalization of EF after medical treatment, but they would still retain their original classification of HFrEF but may also be referred to as having heart failure with improved EF (HFiEF).
Those with an EF >50% with current or previous signs and symptoms of HF are referred to as having heart failure with preserved ejection fraction (HFpEF). This was previously termed diastolic dysfunction. There is also a small subgroup that has an EF between 41% and 49%, and this is called heart failure with midrange ejection fraction (HFmEF). This group has been poorly studied.
This article includes details on how to initiate medications, which side effects are most common and how to deal with them, potential interactions that can occur with multiple medications, monitoring parameters when up-titrating and how medications can interact with different disease states.
Signs and symptoms
Although the causes of HF can be different, the clinical presentation results in similar signs and symptoms of reduced cardiac output and/or fluid overload (Table 2). In addition to signs and symptoms, evaluation of functional capacity is also used to assess the degree of patient disability. The New York Heart Association (NYHA) functional classification (Table 3) is a common, practical tool used to describe the severity of patients’ symptoms. This classification is also frequently used to define HF populations in clinical trials. Pharmacists should be familiar with these and use them in their patient assessments.
Table 2.
| Common | Uncommon |
|---|---|
| Dyspnea Orthopnea Paroxysmal nocturnal dyspnea Fatigue Weakness Exercise intolerance Dependent edema Cough Weight gain Abdominal distension Nocturia Cool extremities |
Cognitive impairment*
Altered mentation or delirium* Nausea Abdominal discomfort Oliguria Anorexia Cyanosis |
Might be more common presentation in elderly patients.
Table 3.
New York Heart Association functional classification and other symptom descriptors
| Class | Definition | Other descriptor |
|---|---|---|
| I | No symptoms | Asymptomatic |
| II | Symptoms with ordinary activity | Mild symptoms |
| III | Symptoms with less than ordinary activity | Moderate symptoms |
| IV | Symptoms at rest or with any minimal activity | Severe symptoms |
Diagnosis
While symptoms such as edema, fatigue and dyspnea are typical manifestations of HF, atypical presentations can occur, and other conditions may have similar symptoms. Therefore, in addition to symptoms, a thorough clinical history and physical examination, along with other investigations, are performed to help rule in or rule out HF. These investigations may include a chest X-ray, echocardiogram, lab work (such as thyroid function, renal function) and natriuretic peptides. Subsequently, all patients with suspected HF should undergo diagnostic imaging to assess the structure and function of the heart. It is recommended that an echocardiogram be performed in all patients to determine left ventricular ejection fraction (among other parameters); however, other modalities may also be used to help assess and determine the specific etiology of HF. The specific approaches to identifying the most common etiologies of HF can be found in the full guideline document.
Prevention
It is recommended that all patients have a clinical assessment to identify known or potential risk factors for the development of HF. Conventional risk factors for cardiovascular disease, such as hypertension, diabetes, obesity, smoking and dyslipidemia, overlap with specific risk factors attributed to the development of HF. Identification and modification of risk factors is key to the prevention of HF. Appendix 1 (available at www.cpjournal.ca) lists additional risk markers for the development of HF.
Pharmacological treatment of HF
The overall goals of therapy in the treatment of HF include improving survival, reducing hospitalization, increasing functional capacity, mitigating symptoms and ultimately improving quality of life.
Heart failure with preserved ejection fraction (HFpEF)
Much of the clinical trial data pertaining to the treatment of HF with pharmacotherapy has focused on HFrEF. While there are a few studies that have looked at the treatment of HF patients with preserved ejection fraction (HFpEF) with similar agents used in HFrEF, overall results have generally been neutral, and hence recommendations are mostly aimed at identifying and treating underlying/predisposing factors (such as hypertension) and controlling symptoms of congestion with diuretics as required.
Heart failure with reduced ejection fraction (HFrEF)
The overall approach to pharmacotherapy has significantly changed in the 2017 guidelines. The last major update to the overall approach of pharmacotherapy occurred in 2012, and several changes incorporating new evidence over the past 5 years have been made. Additionally, new agents used in the treatment of HFrEF have entered the Canadian market (Table 4).
Table 4.
Evidence-based drugs: start and target doses as shown in large clinical trials
| Drug | Starting dose | Titration | Target dose |
|---|---|---|---|
| ACEI | |||
| Captopril | 12.5 mg TID | Titrate every 2-4 weeks | 25-50 mg TID |
| Enalapril | 1.25-2.5 mg BID | 10 mg BID | |
| Lisinopril | 2.5-5 mg daily | 20-35 mg daily | |
| Perindopril | 2-4 mg daily | 4-8 mg daily | |
| Ramipril | 1.25-2.5 mg BID | 5 mg BID | |
| Trandolapril | 1-2 mg daily | 4 mg daily | |
| ARB | |||
| Candesartan | 4-8 mg daily | Titrate every 2-4 weeks | 32 mg daily |
| Valsartan | 40 mg BID | 80 mg BID | |
| Beta-blockers | |||
| Carvedilol | 3.125 mg BID | Titrate more slowly every 4 weeks | 25 mg BID 50 mg BID if >85 kg |
| Bisoprolol | 1.25 mg daily | 10 mg daily | |
| Metoprolol* | 6.25-12.5 mg BID | 100 mg BID | |
| MRA | |||
| Spironolactone | 12.5 mg daily | Titrate every 2-4 weeks | 50 mg daily |
| Eplerenone | 12.5-25 mg daily | 50 mg daily | |
| ARNI | |||
| Sacubitril/valsartan | 50-100 mg BID | Titrate every 3-6 weeks | 200 mg BID |
| If inhibitor | |||
| Ivabradine | 2.5-5 mg BID | Titrate every 2 weeks | Target to heart rate 50-60 bpm Maximum dose: 7.5 mg BID |
| Vasodilators | |||
| Isosorbide dinitrate | 20 mg TID | Titrate every 2-4 weeks | 40 mg TID Equivalent dose: NTG patch ≈ 0.8-1.0 mg/h Isosorbide-5-mononitrate ≈ 60 mg daily |
| Hydralazine | 37.5 mg TID | 75-100 mg TID-QID | |
| Loop diuretics | |||
| Furosemide | 20-40 mg daily-BID | Titrate to euvolemia | 200 mg/day (caution with >120 mg) |
| Bumetanide | 0.5-1 mg daily-BID | 10 mg/day | |
| Ethacrynic acid | 25-50 mg daily- BID | 400 mg/day (200 mg BID) | |
| Thiazide diuretics | |||
| Metolazone | 2.5 mg daily | Titrate to euvolemia | 20 mg/day |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BID, twice daily; MRA, mineralocorticoid receptor antagonist; NTG, nitroglycerin; QID, 4 times daily; TID, 3 times daily.
Limited evidence of short-acting metoprolol tartrate in heart failure (HF); however, metoprolol succinate CR/XL, with which the HF studies were done, is not available in Canada.
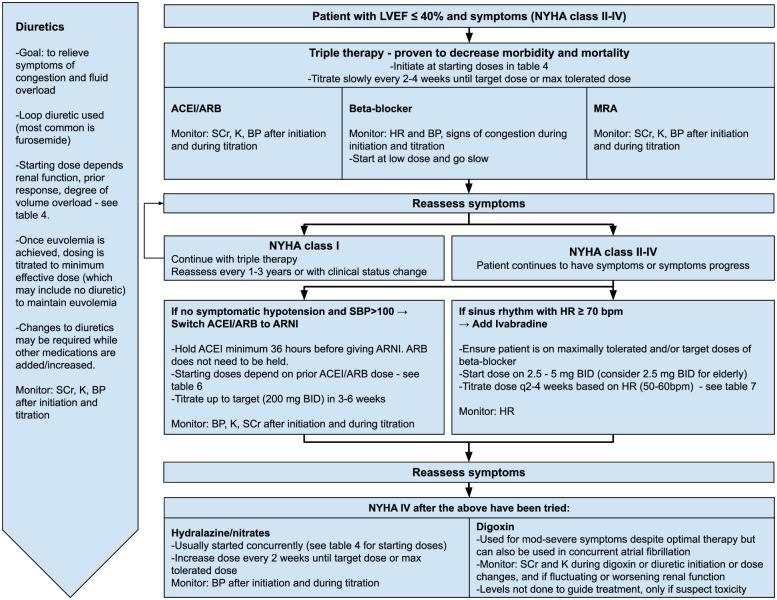
Figure 1 summarizes the treatment recommendations. Described below are highlighted key recommendations, supporting evidence and considerations when applying these to the individual patient with HFrEF. Tables 4 to 8 outline details for each medication class used in the treatment of HFrEF, including practical considerations and tips for monitoring.
Figure 1.
Pharmacologic approach to patients with heart failure and reduced ejection fraction (HFrEF)
Adapted from the 2017 Comprehensive Update of the CCS Guidelines for the Management of Heart Failure.2
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BP, blood pressure; HR, heart rate; K, potassium; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure; BID, twice daily; bpm, beats per minute; q, every; SCr, serum creatinine.
Table 8.
Education for patients: Signs and symptoms of worsening heart failure
| Signs and symptoms of worsening heart failure | Action needed |
|---|---|
| • Weight gain: 1.5 kg in 2 days or 2.5 kg over 1
week • Edema is worsening • Activities are limited due to shortness of breath more than usual • Requiring more pillows to prop them up or needing sleep in chair • Changes in appetite: decreases in appetite can result from edema around the gut • Increased fatigue |
May require medication dose adjustments |
| • Significant weight gain: 2.5 kg in 2
days • Shortness of breath at rest or that does not improve or resolve • Difficulty breathing at night • Extreme fatigue |
May require immediate medical attention |
Most patients with HFrEF should be treated with triple therapy: an angiotensin-converting enzyme inhibitor (ACEI) (or angiotensin receptor blocker [ARB] if ACEI intolerant), a beta-blocker (BB) and a mineralocorticoid receptor antagonist (MRA), unless specific contraindications exist. These agents are titrated to evidence-based target doses or maximum tolerated doses.
Why the change:
Previous iterations of the guidelines recommended initiating dual therapy with ACEI/ARB and BB and only adding an MRA if the patient remained symptomatic with very specific criteria. The 2017 guidelines aimed to simplify the approach to the majority of patients with HFrEF and also to account for data highlighting MRA underutilization.
- Key clinical trials:
- There are no new clinical trials affecting this recommendation.
- The EMPHASIS trial (2012) showed that eplerenone (compared to placebo) decreased the risk of cardiovascular (CV) deaths and hospitalizations due to heart failure in patients with an EF ≤35% and NYHA class II symptoms in addition to good medical management (ACEI/ARB and BB) with a recent CV hospitalization or elevated brain natriuretic peptide (BNP).21
- This data adds to the RALES trial (1999) data and provides evidence that MRAs improve HF outcomes across the spectrum of patients with HFrEF (NYHA classes II-IV).22
How to apply:
Clinical trials evaluating MRAs in HFrEF have excluded patients with glomerular filtration rate (GFR) <30 mL/min/1.73 m2 or serum potassium (K+) >5.0 mmol/L.
MRAs can cause hyperkalemia, especially during acute dehydrating illness with worsening renal function. Close monitoring of serum creatinine and potassium is required. High-risk groups include those with diabetes, preexisting renal dysfunction and older age.
Management approaches to hyperkalemia can be found in the full guideline document (p. 1359).
Triple therapy is typically started in a stepwise approach, depending on the clinical scenario and parameters (e.g., blood pressure [BP], heart rate [HR], serum creatinine [SCr] and K). ACEI/ARBs are generally started prior to an MRA to establish BP, K+ and SCr response; however, target doses of ACEI/ARB do not need to be achieved prior to initiating MRAs.
The goal is to titrate and monitor regularly to achieve target doses (Table 4) or maximally tolerated doses within 6 months.
Considerations when starting each class and what to monitor are outlined in Table 5.
Table 5.
Guideline-directed medical therapy considerations and monitoring
| Drug | Considerations when starting | Monitoring |
|---|---|---|
| ACEI/ARB | -For those unable to tolerate ACEI due to cough or
angioedema, ARBs are a reasonable alternative. - When starting an ACEI, document the presence or absence of cough prior to initiation. -Either ACEI/ARB or BB can be started first depending on multiple clinical factors such as volume status, BP, renal function and acuity. They may be initiated concurrently as well. |
Cough and angioedema
-Cough occurs in 10% to 20% of patients receiving ACEIs and does not require discontinuation unless it is bothersome to the patient. -In patients with angioedema from an ACEI (uncommon, <1%), an ARB can be used cautiously as there have also been reports of angioedema with ARBs. Renal function and serum potassium (K+) - Concurrent MRA therapy requires closer monitoring of K+ and SCr. -Watch for trends in the K+. If increasing, the patient may need adjustment of contributing factors such as diet and medications to preemptively prevent hyperkalemia. -There are no significant differences between ACEIs and ARBs for hyperkalemia, renal dysfunction or BP to warrant switching between agents. -A change in SCr of up to 30% is acceptable with the introduction of an ACEI/ARB. There is no immediate need to decrease the drug dose if the increase in SCr stabilizes, but closer long-term monitoring should be considered. -Monitor SCr and K+ 1 to 2 weeks after initiation and dose increases. Blood pressure - Exaggerated BP lowering may occur if starting at high doses or combined with diuretic therapy. |
| Beta blockers | -Should start at very low doses and titrate slowly to
prevent decompensation or worsening of HF
initially. -Those in NYHA classes I to II can be safely initiated by a nonspecialist physician. For NYHA classes III to IV, a BB should be initiated by a specialist in HF management and have close follow-up. -In reactive airway disease, use a selective BB cautiously (e.g., bisoprolol or metoprolol). -Reassess the need for other AV nodal blocking drugs used in combination (e.g., digoxin, amiodarone). If heart block is present, these may need to be minimized or stopped. |
Heart rate and blood pressure
-Consider a dose reduction if HR less than 50. -If the patient has a low HR (HR 50-60) but is asymptomatic, there is no need to decrease dose. -Carvedilol, due to its α-1 blockade, will decrease blood pressure more than a beta-selective BB. If low BP is an issue, consider using a beta-selective BB. Fluid retention -Transient fluid retention can occur when increasing BB and patient may require a diuretic dose change. |
| MRA | -Usually started after ACEI/ARB and BB have been introduced (but do not have to be at target doses prior to MRA initiation). |
Renal function and electrolytes
-Monitor K+ and SCr 2 to 3 days after initiation, then 1 week after initiation. Check monthly ×3 months and then every 3 months thereafter. More frequent monitoring may be required in acute illness and those at high risk (DM, CKD, older). Hormonal side effects - Eplerenone, a more selective mineralocorticoid receptor antagonist, can be considered in those experiencing hormone-related side effects (e.g., gynecomastia [9%], impotence, postmenopausal bleeding) from spironolactone. |
| ARNI | -Consider for patients who continue to be symptomatic (NYHA
II symptoms or greater) despite being on triple
therapy. -Avoid in patients who have a K >5.2 mmol/L, eGFR <30 mL/min and symptomatic hypotension with a systolic BP <100 mmHg (PARADIGM-HF exclusion criteria). |
Side effects and tolerability similar to
ACEI/ARB
If patient has angioedema to ACEI or ARB, ARNI is contraindicated. Renal function and electrolytes Monitor SCr and K+ 1 week after initiation or dose increase; after dose stabilization, monitor every 3 months. Blood pressure ARNI can have more of a hypotensive effect compared to ACEI/ARBs. |
| If inhibitor | -Consider for patients who continue to be symptomatic (NYHA
II symptoms or greater) despite being on appropriate doses
of triple therapy and have a heart rate >70
bpm. -Because ivabradine works on the SA node, the patient has to be in normal sinus rhythm (e.g., cannot have atrial fibrillation, sick sinus syndrome without pacemaker or pacemaker dependence). -Patient should not be in acute decompensated HF or have a BP <90/50 mmHg. |
Heart rate
Both ivabradine and BB will decrease HR. BB doses are optimized to target doses or maximally tolerated doses prior to starting ivabradine, so ivabradine is intended to further decrease HR to a target of 50 to 60 bpm. Monitoring HR and adjusting the ivabradine dose every 2 weeks may be required. -Ivabradine has no adverse effect on blood pressure in stable ambulatory patients. Interactions -Contraindicated with strong CYP3A4 inhibitors. |
| Vasodilators | -Hydralazine and ISDN are used in combination. -Used in addition to standard treatment for black patients with HFrEF already on triple therapy at appropriate doses and who have advanced symptoms. -Used if the patient is unable to tolerate ACEI/ARB/ARNI (significant worsening renal function or persistent hyperkalemia despite dose reductions of RAAS agent, elimination and modification of other contributing factors). |
Blood pressure
Tolerance/administration -Avoid continuous (24-hour) use of nitrates because patients can develop tolerance. |
| Digoxin | -Used in HFrEF with moderate to severe symptoms despite on
appropriate doses of medications. -Digoxin may also be used for atrial fibrillation refractory to BB. |
Electrolytes and kidney function
-Monitor K+ and SCr when changing digoxin or diuretic dose or during dehydrating illness. -Increased monitoring may be required if the patient has decreasing or fluctuating renal function, is elderly or has low body weight. -Digoxin levels are not done routinely and are not used to guide treatment. Levels are only done if one is suspecting toxicity. |
| Diuretics | -Diuretics are used to manage symptoms of fluid overload and
congestion. -Loop diuretics such as furosemide are most commonly used. -Starting dose will depend on patient symptoms, degree of volume overload, renal function and prior response to diuretics. Doses may need to be higher for lower renal function. -The dose of the diuretic may need to be decreased when increasing doses of ACEI/ARB/ARNI. -If requiring high doses of furosemide (≥120 mg/day), consider adding a thiazide diuretic such as low-dose metolazone as long as close monitoring of daily weights, renal function and serum potassium is possible. |
Electrolytes and kidney function
-Monitor SCr and electrolytes 2 to 3 days, then 1 week after initiation and dose changes. Blood pressure Weight -Daily morning weights should be monitored in patients with HF with fluid retention or congestion that is not easily controlled with diuretics or in patients with significant renal dysfunction. -Monitor weight more closely in unstable or frail patients: rapid weight gain (1.5-2 kg) should prompt a rapid medical visit. Symptoms -Diuretics are titrated to symptom relief. When euvolemia is achieved, dosing is titrated to the minimum effective dose to maintain euvolemia, which may include no diuretic at all. -Some patients may be managed with a diuretic as needed and given parameters as to when the diuretic should be taken (e.g., take 20 mg of furosemide when >1 kg gained in 3 days). |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; AV, atrioventricular; BB, beta-blocker; BP, blood pressure; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, having heart failure with reduced ejection fraction; ISDN, isosorbide dinitrate; K, potassium; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RAAS, renin-angiotensin-aldosterone system; SA, sinoatrial; SCr, serum creatinine.
In patients who continue to be symptomatic after optimization of triple therapy, consider switching the ACEI/ARB to an angiotensin receptor neprilysin inhibitor (ARNI).
Why the change:
Sacubitril/valsartan is an ARNI and currently is the only agent of its class available on the Canadian market. Sacubitril inhibits the neprilysin enzyme, resulting in increased natriuretic peptides. Natriuretic peptides promote diuresis, vasodilation, aldosterone suppression and inhibit fibrosis. Use of a neprilysin inhibitor is aimed at augmenting the beneficial physiological neurohormonal effects that occur in HF, which is in contrast to other agents used in HFrEF, which inhibit the deleterious neurohormonal effects of HF.
- Key clinical trials:
- There are no new clinical trials affecting this recommendation; however, the agent entered the Canadian market in 2015.
- The PARADIGM-HF (2014) was a landmark trial comparing sacubitril/valsartan to enalapril in patients with NYHA class II to IV symptoms, left ventricular ejection fraction (LVEF) ≤40% and a BNP ≥150 pg/mL or N-Terminal Prohormone B-type Natriuretic Peptide (NT-ProBNP) ≥600 pg/mL (or if they were hospitalized in the last year for HF, a BNP ≥ 100 pg/mL or NT-ProBNP ≥400 pg/mL).23
- ■ Patients were eligible for study inclusion if they were on a moderate-dose ACEI/ARB (equivalent to enalapril 10 mg) and BB therapy for at least 4 weeks. Therapy with MRA was recommended but not required.
- ■ Sacubitril/valsartan decreased the primary composite outcome of death from CV cause and hospitalization for HF by 20% when compared to enalapril. (Number needed to treat [NNT] of 21 over 2.25 years to prevent 1 CV death or HF hospitalization.)
- ■ Symptoms and physical limitations were also significantly decreased for patients in the sacubitril/valsartan arm.
How to apply:
Table 6 provides a suggested initial dose depending on the patient’s prior ACEI or ARB dose.
- Switching between agents needs some consideration.
- ACEI to/from ARNI:
- ■ For patients on an ACEI, there should be at least a 36-hour washout period prior to starting the ARNI. A washout period is required to decrease the risk of angioedema.
- ARB to/from ARNI:
- ■ A washout period is not required.
Due to the inclusion criteria of an elevated BNP/NT-ProBNP in the PARADIGM-HF trial, certain jurisdictions require a BNP/NT-ProBNP level to determine eligibility for drug coverage.
- Compared to enalapril, patients on sacubitril/valsartan experienced more hypotension and nonserious angioedema. However, they experienced less renal impairment, hyperkalemia, cough and discontinuations due to adverse events.
- Blood pressure and signs and symptoms of hypotension should be monitored more closely when switching agents.
ARNIs promote natriuresis, and therefore reassessment of diuretic dose should be considered upon initiation and up-titration.
Table 6.
Potential valsartan/sacubitril dosing and titration2
| Higher dose of RAAS inhibitor (mg/day) | Initial dose | Titration | |
|---|---|---|---|
| Enalapril ≥10 Lisinopril ≥10 Perindopril ≥4 Ramipril ≥5 |
Candesartan ≥16 Irbesartan ≥150 Losartan ≥50 Olmesartan ≥10 Telmisartan ≥ 40 Valsartan ≥160 |
100 mg PO BID | Over 3-6 weeks, increase to target 200 mg PO BID |
| Lower dose of RAAS inhibitor | 50-100 mg PO BID | Over 6 weeks, increase to target 200 mg PO BID | |
| High risk of hypotension (low baseline SBP, poor renal function) | 50 mg PO BID | ||
BID, twice daily; PO, orally; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; SCr, serum creatinine.
In patients who continue to be symptomatic after optimization of triple therapy, who are in sinus rhythm with a resting heart rate >70 bpm and have had a HF hospitalization within the past 12 months, consideration should be given to the addition of ivabradine.
Why the change:
Previous trials of BBs in HF have shown a correlation between lower heart rate and improved HF outcomes. It is hypothesized that perhaps the beneficial effects of BBs in HF are related to a reduced heart rate, which allows for better filling and contraction of the ventricles. Ivabradine selectively inhibits the If current (or funny channel) in the sinus node, which slows depolarization, decreasing heart rate and prolonging diastolic time. This, in turn, increases stroke volume while still preserving myocardial contractility and relaxation, with no direct effect on blood pressure.
Ivabradine is the first of its class and currently the only agent of its class available on the Canadian market.
- Key clinical trial:
- There are no new clinical trials affecting this recommendation; however, ivabradine entered the Canadian market in 2017.
- The SHIFT trial (2010) compared ivabradine to placebo in patients with LVEF ≤35%, NYHA class II to IV symptoms, on good medical management (ACEI/ARB + BB ± MRA), hospitalized for HF within the last year and in sinus rhythm with a heart rate >70 bpm.24
- ■ Ivabradine decreased the primary outcome (CV death and HF admission) by 18%, which was largely driven by hospital admission for worsening HF (NNT = 20 in 1.9 years).
How to apply:
Every effort should be made to achieve target/maximum tolerated dose of BB before initiating ivabradine. Continue the BB when starting ivabradine.
Start ivabradine at 5 mg twice a day, but consider the lower dose of 2.5 mg twice a day for the elderly or those with a history of conduction defects.
Adjust ivabradine every 2 weeks to target HR of 50 to 60 bpm (see Table 7). Unlike BBs, there is no target dose.
A subgroup analysis showed that the primary composite endpoint did not reach significance for patients with a baseline HR <77 bpm; therefore, regulatory bodies have approved the use of ivabradine in minimum heart rates ranging from 70 to 77 bpm. Health Canada’s official indication is at a HR above 77 bpm.
- Symptomatic bradycardia and visual anomalies (phosphenes) occurred more frequently in the ivabradine group compared to the placebo group.
- For symptomatic bradycardia, dose adjustments or discontinuation may be required (see Table 8).
- Patients experiencing phosphenes should be cautioned about driving, especially at night. Phosphenes appear to be a transient side effect.
Table 7.
Suggested ivabradine dose titration
| Resting heart rate | Dose adjustment every 2 weeks |
|---|---|
| >60 bpm | Increase dose by 2.5 mg BID, to a maximum dose of 7.5 mg BID. |
| 50-60 bpm | Maintain at current dose |
| <50 bpm | Decrease dose by 2.5 mg BID. If current dose is 2.5 mg BID, discontinue ivabradine. |
BID, twice daily.
Combination of ACEI and ARB is no longer recommended.
Why the change:
While there is a small benefit to combination ACEI and ARB in HF (mainly HF hospitalizations), other data indicate that the combination is associated with high rates of hypotension, hyperkalemia and renal dysfunction, pushing the risks to outweigh the benefits.25
Given newer, more robust evidence for the benefits of adding an MRA and an ARNI, there is now little role for combination ACEI and ARB therapy.
How to apply:
ACEI and ARB combination therapy should be discontinued and optimization of other guideline medications should be pursued according to the treatment algorithm.
Hydralazine with isosorbide dinitrate (H-ISDN) and digoxin may still be considered but only in those who continue to be NYHA classes III to IV after all the above is tried.
H-ISDN may be also considered in those who cannot tolerate ACEI/ARB/ARNI due to renal dysfunction.
Why the change:
With the arrival of new therapies and newer evidence, these medications offer minimal benefit in comparison to contemporary therapy. They are now considered last line after all other therapies have been tried if a patient continues to be severely symptomatic.
How to apply:
- These medications are added in addition to the other medications, except for in the case of substituting the ACEI/ARB/ARNI with H-ISDN for those who cannot tolerate ACEI/ARB/ARNI:
- Patients with a significant change in baseline SCr with ACEI/ARB/ARNI therapy (over 30% increase) that persists despite modification of dose, rechallenge and/or removal of other potentially nephrotoxic agents
- Patients with a SCr >220 µmol/L who experience worsening renal function with the use of ACEI/ARB/ARNI
- Patients with persistent hyperkalemia despite changes in diet, dose reduction of ACEI/ARB/ARNI and/or removal of other agents that can increase potassium levels
Addition of H-ISDN to standard HF medication (ACEI/ARB + BB + MRA) may be considered in black patients.
Isosorbide mononitrate and transdermal nitroglycerin are often used in place of isosorbide dinitrate (ISDN) due to ease of use (once-daily dosing) and availability, although ISDN is the only nitrate studied in HF trials.
Dealing with general side effects
Side effects can be common for patients with HF due to the prevalence of multiple comorbidities and medications. Below are ways to manage some of the most common side effects:
Hypotension
If a patient is experiencing symptomatic hypotension (systolic blood pressure [SBP] <100 mmHg + symptoms of hypotension), reevaluate other antihypertensives without mortality benefit in HF and decrease/discontinue those agents before altering guideline-directed medical therapy (GDMT). Reassessment of diuretic doses should also be done.
Try separating or staggering administration of drugs that lower BP (e.g., BB in the morning and ACEI/ARB in the evening) if patient has symptomatic hypotension.
Patients with HF may have low blood pressure due to the reduced ejection fraction. This should not be an indication to lower doses of GDMT unless the patient is symptomatic and other strategies (such as the ones listed above) have been tried.
Hyperkalemia due to renin-angiotensin-aldosterone system inhibitors
- For all instances of hyperkalemia:
- Review all medications, and ensure there are no other causes or sources of hyperkalemia (e.g., potassium-sparing diuretics, potassium supplements).
- Consider renal function and medications that may worsen renal function and hyperkalemia (e.g., NSAIDs, COX-2, SGLT-2 inhibitors).
- Suggest dietary potassium restriction, and refer to a dietitian.
- Watch for trends and rapid changes in potassium.
For more details on the management of hyperkalemia, please refer to the full Canadian Cardiovascular Society (CCS) 2017 guidelines, Table 13.2
Renal dysfunction due to renin-angiotensin-aldosterone inhibitors
An increase in SCr or decrease in estimated GFR (eGFR) of up to 30% is not unexpected when an ACEI/ARB is introduced; if the increase stabilizes at 30%, there is no immediate need to decrease the drug dose, but closer long-term monitoring might be required.
Consider concomitant medications that can also worsen renal function (e.g., NSAIDs, COX-2 inhibitors, SGLT-2 inhibitors).
Consider volume status. Volume depletion or dehydration due to diuretics can worsen renal function. Fluid overload from HF can also decrease kidney perfusion and hence increase SCr. Look at the whole clinical picture.
If the SCr increases more than 30%, a decreased dose or rechallenge when the patient is more stable may be required. If the patient still experiences significant renal dysfunction, consider the combination of hydralazine and ISDN in place of the renin-angiotensin-aldosterone (RAAS) agent.
Not recommended
Statins, antiplatelets and anticoagulation are not recommended specifically for HFrEF unless there is another indication present. Anti-inflammatories, calcium channel blockers (except amlodipine) and anti-arrhythmics (except amiodarone) should be avoided (Appendix 2).
Pharmacists should routinely inquire about NSAID/COX-2 inhibitor use, especially those available for self-selection over the counter. These agents promote sodium and water retention, worsening renal function (especially in combination with a RAAS inhibitor), elevate blood pressure and have been shown to increase CV events and worsen HF.
Considerations with other disease states and drugs
Atrial fibrillation
Atrial fibrillation (AF) can exacerbate HF by decreasing cardiac output, increasing myocardial oxygen consumption and decreasing coronary perfusion, so controlling it is important for the management of HF.
- Rate control is recommended for patients with HF and AF.
- Beta Blockers: BBs are recommended for rate control. Caution when up-titrating is still required, especially in those who are fluid overloaded.
- Calcium channel blockers (CCBs): CCBs should not be used in HFrEF patients with AF due to the risk of worsening cardiac function. They can be considered for patients with HFpEF.
- Digoxin: For patients with poor rate control on a BB, digoxin is recommended to be used in addition to the BB.
- ■ For patients who cannot tolerate/use BBs, digoxin is recommended in place of a BB for rate control.
- ■ Digoxin levels do not need to be done routinely, only if toxicity is suspected. If a digoxin level is done, trough serum digoxin levels should not exceed 1.0 ng/mL.
- ■ Digoxin dose should be adjusted for renal function and titrated to HR control (<100 bpm).26
- If rhythm control is required:
- Amiodarone: Amiodarone should be used if rhythm control is required.
- Avoid:
- ■ Dronedarone should not be used in patients with HFrEF or recent decompensation of their HF.
- ■ Avoid sotalol, flecainide and propafenone.
Oral anticoagulation should be started for all patients with HF and AF unless contraindicated as per CCS AF guidelines.
Diabetes
- Diabetes agents in HF:
- Metformin: Metformin is still considered first-line therapy for type 2 diabetes (T2DM) with HFrEF.
- SGLT-2 inhibitors: SGLT-2 inhibitors such as canagliflozin, empagliflozin and dapagliflozin have been shown to improve outcomes such as preventing HF hospitalization and CV mortality, largely in primary prevention of HF.27,28 They are a reasonable choice to use after metformin in patients with both T2DM and HF.
- ■ SGLT-2 inhibitors cause osmotic diuresis due to the increased secretion of glucose in the urine. When used in conjunction with other diuretics, they can have an additive volume reduction.
- ■ Patients who are on SGLT-2 inhibitors should have their volume status and kidney function monitored more closely, and diuretic dosing may need to be adjusted accordingly.
- ■ The safety and efficacy of these agents are currently being tested in patients with established HFrEF on optimal HF therapy.
- Thiazolidinediones: Thiazolidinediones (such as pioglitazone and rosiglitazone) have been shown to increase the risk of HF and are not recommended for patients with HF.
- DPP-4 inhibitors: Saxagliptin was found to increase hospitalization for HF; therefore, use cautiously in those with HF.29
- ■ Other DPP-4 inhibitors did not reflect the same result.
- GLP-1 agonists: While liraglutide has been shown to have positive CV outcomes, it has not shown any benefit specifically for HF.
- ■ Other GLP-1 agonists did not have any effect on HF outcomes.
- HF agents in diabetes:
- Patients with diabetes are at increased risk of renal dysfunction and may warrant closer monitoring of their renal function and electrolytes when started on RAAS-blocking agents.
Chronic kidney disease
- HF medications in chronic kidney disease (CKD):
- For HF patients with stable mild-to-moderate CKD (GFR >30 mL/min), ACEIs/ARBs and MRAs should be considered.
- Some HF medications are renally cleared and may need to be adjusted or avoided in renal dysfunction and ADRs (Appendix 3).
- Other considerations:
- For patients with CKD and HF, monitoring of electrolytes and creatinine should be more frequent, especially in acute illness, dehydration and titration of cardiac medications, including diuretics.
- While overdiuresis and dehydration from a diuretic can cause acute kidney injury (AKI) and a rise in SCr, diuretics are not contraindicated in CKD or even in some settings of AKI.
- ■ Patients who have CKD may require higher doses of loop diuretics or an addition of another diuretic (e.g., metolazone) to elicit an adequate response.
- ■ Renal dysfunction may also be due to HF and volume overload, which causes decreased perfusion to the kidneys. In this scenario, diuretics should be used to relieve congestion, which will help improve kidney function.
Nonpharmacological management
Much of the recommendations for nonpharmacological management have remained the same. Appendix 4 describes nonpharmacological management that most patients with HF should be encouraged to follow.
Patient education and resources/tools
Education is important for the patients to manage their symptoms, prevent hospitalizations and prevent progression of their disease. Patients should be educated on self-monitoring and when they should seek medical attention (Table 8).
Pharmacists should recommend that daily weigh-ins be done in HF patients with fluid retention, poorly controlled congestion or significant renal dysfunction. Patients should weigh themselves daily in the morning, after voiding, to monitor for fluid retention as a sign of worsening HF. Additional resources can be found in Appendix 5.
Supplemental Material
Supplemental material, 853307_Appendix_1_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental Material
Supplemental material, 853307_Appendix_2_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental Material
Supplemental material, 853307_Appendix_3_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental Material
Supplemental material, 853307_Appendix_4_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental Material
Supplemental material, 853307_Appendix_5_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Footnotes
Declaration of Conflicting Interests:LB and SK declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article. JAE has received grants or honoraria from Novartis, Servier, Bayer, Pfizer, Merck, Trevena, Amgen, Canadian Institutes of Health Research, National Institutes of Health and Heart and Stroke Foundation of Canada.
Author Contributions:SK initiated the project; SK and LB were responsible for the design and methodology; LB drafted the first draft; all critically reviewed draft manuscript; all reviewed the final draft; SK supervised the project.
Funding:The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Heart and Stroke Foundation of Canada. 2016 Report on the health of Canadians. Available: www.heartandstroke.ca/-/media/pdf-files/canada/2017-heart-month/heartandstroke-reportonhealth-2016.ashx?la=en&hash=91708486C1BC014E24AB4E719B47AEEB8C5EB93E (accessed April 16, 2019).
- 2. Ezekowitz J, O’Meara E, McDonald M, et al. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017;33:1342-433. [DOI] [PubMed] [Google Scholar]
- 3. Gunadi S, Upfield S, Pham ND, Yea J, Schmiedeberg MB, Stahmer GD. Development of a collaborative transitions-of-care program for heart failure patients. Am J Health Syst Pharm 2015;72:1147-52. [DOI] [PubMed] [Google Scholar]
- 4. Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007;146:714-25. [DOI] [PubMed] [Google Scholar]
- 5. Davis EM, Packard KA, Jackevicius CA. The pharmacist role in predicting and improving medication adherence in heart failure patients. J Manag Care Pharm 2014;20(7):741-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med 1998;158:1067-72. [DOI] [PubMed] [Google Scholar]
- 7. Eggink RN, Lenderink AW, Widdershoven JW, van den Bemt PM. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci 2010;32:759-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007;146:714-25. [DOI] [PubMed] [Google Scholar]
- 9. Lopez Cabezas C, Salvador C, Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp 2006;30:328-42. [DOI] [PubMed] [Google Scholar]
- 10. Jackevicius CA, de Leon NK, Lu L, Chang DS, Warner AL, Mody FV. Impact of a multidisciplinary heart failure post-hospitalization program on heart failure readmission rates. Ann Pharmacother 2015;49(11):1189-96. [DOI] [PubMed] [Google Scholar]
- 11. Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy 1999;19(7):861-9. [DOI] [PubMed] [Google Scholar]
- 12. Roughead EE, Barratt JD, Ramsay E, et al. The effectiveness of collaborative medicine reviews in delaying time to next hospitalization for patients with heart failure in the practice setting: results of a cohort study. Circ Heart Fail 2009;2(5):424-8. [DOI] [PubMed] [Google Scholar]
- 13. Coons JC, Fera T. Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure. Am J Health Syst Pharm 2007;64:1274-8. [DOI] [PubMed] [Google Scholar]
- 14. Murray MD, Ritchey ME, Wu J, Tu W. Effect of a pharmacist on adverse drug events and medication errors in outpatients with cardiovascular disease. Arch Intern Med 2009;169:757-63. [DOI] [PubMed] [Google Scholar]
- 15. Haynes KT, Oberne A, Cawthon C, Kripalani S. Pharmacists’ recommendations to improve care transitions. Ann Pharmacother 2012;46:1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggink RN, Lenderink AW, Widdershoven JW, vanden Bemt PM. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci 2010;32:759-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez AS, Saef J, Paszczuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm 2013;70:1070-6. [DOI] [PubMed] [Google Scholar]
- 18. Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med 1999;159:1939-45. [DOI] [PubMed] [Google Scholar]
- 19. Jain A, Mills P, Nunn LM, et al. Success of a multidisciplinary heart failure clinic for initiation and up-titration of key therapeutic agents. Eur J Heart Fail 2005;7(3):405-10. [DOI] [PubMed] [Google Scholar]
- 20. Hale GM, Hassan SL, Hummel SL, Lewis C, Ratz D, Brenner M. Impact of a pharmacist-managed heart failure postdischarge (bridge) clinic for veterans. Ann Pharmacother 2017;51(7):555-62. [DOI] [PubMed] [Google Scholar]
- 21. Zannad F, McMurray JJ, Krum H; et al. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364(1):11-21. [DOI] [PubMed] [Google Scholar]
- 22. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341(10):709-17. [DOI] [PubMed] [Google Scholar]
- 23. McMurray JJ, Packer M, Desai AS; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993-1004. [DOI] [PubMed] [Google Scholar]
- 24. Swedberg K, Komajda M, Böhm M; et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376(9744):875-85. [DOI] [PubMed] [Google Scholar]
- 25. Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. J Card Fail 2008;14:181-8. [DOI] [PubMed] [Google Scholar]
- 26. Verma A, Cairns JA, Mitchell LB; et al. CCS Atrial Fibrillation Guidelines Committee. 2014 Focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2014;30(10):1114-30. [DOI] [PubMed] [Google Scholar]
- 27. Zinman B, Wanner C, Lachin JM; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373(22):2117-28. [DOI] [PubMed] [Google Scholar]
- 28. Neal B, Perkovic V, Mahaffey KW; et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377(7):644-57. [DOI] [PubMed] [Google Scholar]
- 29. Scirica BM, Bhatt DL, Braunwald E; et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317-26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 853307_Appendix_1_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental material, 853307_Appendix_2_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental material, 853307_Appendix_3_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental material, 853307_Appendix_4_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada
Supplemental material, 853307_Appendix_5_online_supp for 2017 Guidelines for the management of heart failure by pharmacists by Lesley C. Beique, Justin A. Ezekowitz, Eileen O’Meara, Michael McDonald and Sheri L. Koshman in Canadian Pharmacists Journal / Revue des Pharmaciens du Canada