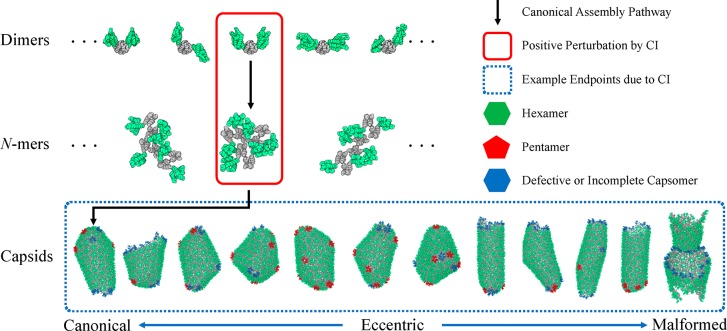
Figure 1.
Schematic of the hierarchical process that is central to HIV-1 mature capsid assembly. Within each oligomeric state of N monomers (N-mer), a variety of configurations are possible. The canonical assembly pathway relies on constant self-correction across N-mer states, which is contingent on a dynamic and broad population of small N-mer intermediates. In this work, we effectively introduce the presence of capsid inhibitor (CI) drugs using a small but fixed population of trimers of dimers, a 6-mer with up to three bound CIs (one at each dimer–dimer interface), thereby perturbing the natural dynamics of the assembly process. Snapshots of the final structures from 12 CG-MD simulations are depicted, from which a majority population of eccentric or malformed capsids can be seen. Here, eccentric (canonical) end-points refer to structures with regions of densely accumulated pentamers and defective hexamers (broadly distributed pentamers) while malformed assemblies are non-enclosed and semi-amorphous structures.