Figure 4.
PKA-Dependent Protein Phosphorylation Deficits Are Shared by Human and Rodent mHTT-Expressing MSNs
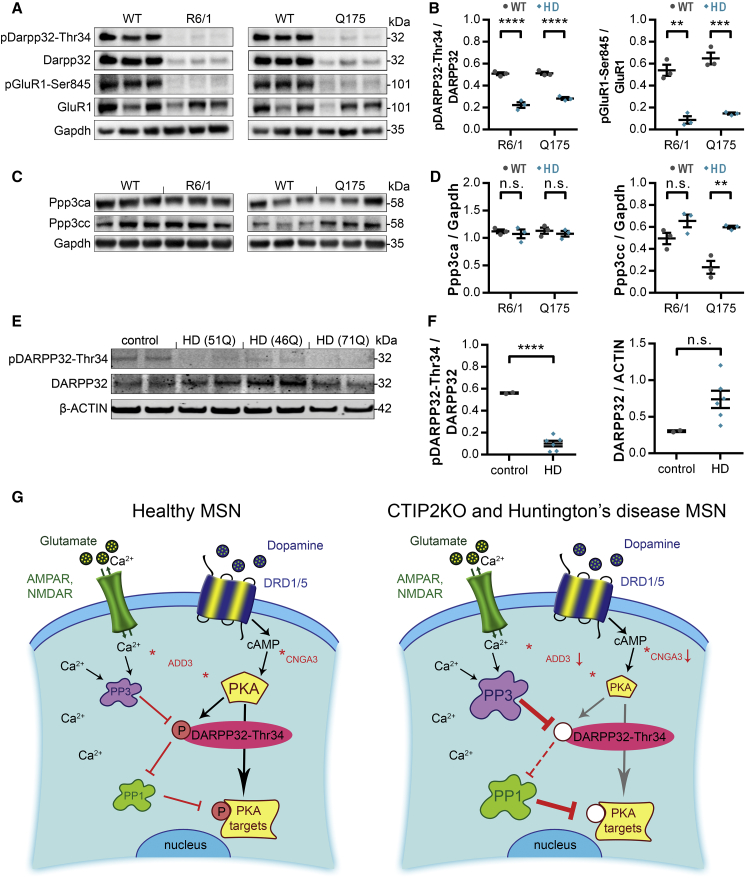
(A and B) Images (A) and quantification (B) showing a significant mHtt-dependent decrease in pDarpp32-Thr34 and pGluR1-Ser845 levels in R6/1 and Q175 HD mouse models (B) (n = 3, 3).
(C and D) Images (C) and quantification (D) of two catalytic subunits of Pp3, Ppp3ca and Ppp3cc, showing a mHtt-dependent increase in Ppp3cc levels in R6/1 and Q175 mice (D) (n = 3, 3).
(E and F) Images (E) and quantification (F) showing a great mHtt-dependent decrease in pDARPP32-Thr34 levels in MSNs derived from three independent HD hPSC lines (F) (n = 2, 6, 2).
(B, D, and F) One-way ANOVA; ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; n.s., not significant. Data are presented as mean ± SEM.
(G) Some of the CTIP2-regulated intracellular events are depicted in a healthy (left) versus CTIP2KO and HD (right) MSN. CTIP2 target genes in the PKA gene set are shown in red and some of their interactions with members of the PKA signaling pathway are indicated with an asterisk. Decreased PKA signaling in CTIP2KO neurons is likely mediated through lower levels of catalytic subunits of PKA available and a loss of pDARPP32-Thr34-regulated inhibition of phosphatase PP1. Most of these molecular changes are also present in mHTT-expressing cells, suggesting that CTIP2 hypofunction might contribute to selective MSN pathology in HD.