Figure 3.
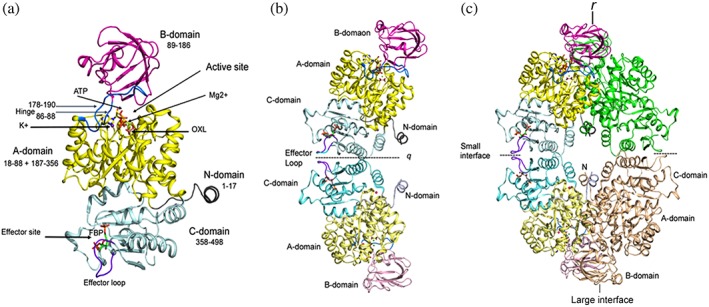
Domain organization and assembly of PyK. (a) Cartoon drawing of LmPyK monomer with domains colored as N (residues 1–17; gray), A (18–88 and 187–356; yellow), B (89–186; magenta), C (358–496; light cyan). Ligands ATP and oxalic acid (OXL) are shown as stick models. Metal ions (Mg2+ and K+) shown as green and purple spheres in the active site are indicated with an arrow. Hinges (86–88 and 178–190) are shown in marine blue. Effector loop (481–487) is shown in dark purple; FDP bound in the effector site is shown as stick model. The LmPyK structure (3HQP) containing ATP, OXL, FDP, Mg2+, and K+ was used in preparing this cartoon. (b) Cartoon drawing showing the LmPyK dimer and the C–C interface also known as the “small interface.” Domains in both subunits are colored similar to those in Figure 2a. Effector loops from each monomer are at the dimer interface. Dimer axis is designated q. (c) Cartoon drawing showing assembly of the LmPyK tetramer in 3HQP. The small and large interfaces are indicated. The large interface is formed by interactions of residues in the A‐domains of neighboring subunits across r axis
