Abstract
Four commercial transport systems for the recovery of Neisseria gonorrhoeae were evaluated in support of the need to obtain culture isolates for the detection of antimicrobial resistance. Bacterial recovery from the InTray GC system was superior with minimal loss of viability in contrast to non-nutritive transport systems.
Keywords: Gonorrhea, Transport, Viable
Microbiologic detection of antimicrobial resistant bacteria varies from traditional assessment of growth dynamics in the presence of antimicrobial agents to direct detection of genetic mutations associated with resistance in clinical specimens. The application of genetic analysis to detect antimicrobial resistance has extraordinary potential to decrease the time required for antimicrobial susceptibility results since it’s not dependent on bacterial growth but is limited by the requirement for well characterized and conserved mutations. N. gonorrhoeae resistance to ciprofloxacin, a previously recommended antibiotic for the treatment of gonorrhea, is conferred by mutations in the gyrA and parC genes which have been targeted for direct detection of resistance (Siedner et al., 2007; Magooa et al., 2013; Nicol et al., 2015). However, ciprofloxacin is no longer recommended in the United States due to established resistance among N. gonorrhoeae isolates (Centers for Disease Control and Prevention, 2015), and mutations associated with resistance to currently recommended combination therapy of ceftriaxone and azithromycin have yet to be sufficiently characterized for diagnostic test development (Unemo & Shafer, 2014; Peterson & Martin, 2015). Culture isolation of N. gonorrhoeae for antimicrobial susceptibility testing is critical for conducting surveillance to detect emerging resistance and help evaluate potential treatment failures (Centers for Disease Control and Prevention, 2014). N. gonorrhoeae is a facultative Gram-negative bacteria that has a low tolerance for survival in varied temperature following clinical specimen collection and transport. Nutritive and non-nutritive transport systems are inoculated with clinical specimens and are typically transported to the laboratory within 24 hours for optimal recovery. However, recovery rates may vary and there are few comparative studies with clinical isolates (Farhat et al., 2001; Wade & Graver, 2003; Arbique et al., 2000; Van Horn et al., 2008; Wade & Graver, 2005; Graver & Wade, 2004). Here we evaluate the recovery of N. gonorrhoeae from 4 commercial transport systems.
Six N. gonorrhoeae isolates collected from men with urethral discharge in 2013 were selected based on antimicrobial susceptibly patterns determined by agar plate dilution. Isolates 1 (PenRTetRCipR-1) and 2 (PenRTetRCipR-2) were resistant, as defined by the Clinical Laboratory Institute Standards (CLSI), to penicillin, tetracycline and ciprofloxacin (Clinical Laboratory Standards Institute, 2015). The ceftriaxone and cefixime minimum inhibitory concentration (MIC) values for isolates 3 (CroDSCfmDS-3) and 4 (CroDSCfmDS-4) were 0.125 μg/mL and 0.25 μg/mL, respectively. Isolates 5 (AziC-5) and 6 (AziC-6) had azithromycin MIC values greater than or equal to 16 μg/mL. All isolates were grown on chocolate agar for 24 h at 5% CO2 and suspended in trypticase soy broth containing 20% glycerol for storage at −70 °C and maintained in a frozen state using dry ice during shipment to 2 state and one local public health laboratories in the United States. A fresh culture of each isolate was prepared as before and serially diluted in 0.85% sterile saline to achieve concentrations of 104–108 colony forming units per mL (CFU/mL). Swabs from the BD CultureSwab W/Media Collection and Transport System (Becton Dickinson Diagnostics Systems, Sparks, MD), BD CultureSwab MaxV Collection and Transport System (Becton Dickinson Diagnostics Systems, Sparks, MD), Copan Liquid Amies Elution Swab (Eswab) Collection and Transport System (Copan Diagnostics Inc., Murrieta, CA) and cotton tipped swabs for use with the InTray GC (Biomed Diagnostics Inc., White City, OR) were separately placed into each of the 5 dilutions for 10 seconds. The BD CultureSwab W/Media, BD CultureSwab MaxV and Copan Eswab were used to inoculate liquid Amies media, respectively. The InTray GC plates were pre-warmed to room temperature (approximately 25 °C) and inoculated with 25 μL of the diluted suspensions. Inoculated liquid Amies media were held at either 4 °C or room temperature for up to 72 h. One set of InTray GC plates was incubated for 24 h at 35 °C and then held at room temperature or 4 °C for up to 72 h. Another set of InTray GC plates was treated in the same manner except that the plates were not incubated at 35 °C following inoculation but rather held at room temperature or 4 °C. Immediately following inoculation and at 24-h intervals, the concentration of viable bacteria in liquid Amies medium was determined by serial dilution onto Modified Thayer Martin (MTM) plates, whereas the number of colonies on each InTray GC plate was enumerated. All procedures were performed at collaborating state public health laboratories and data was analyzed at the Centers for Disease Control and Prevention. The CFU/mL for each isolate, highest dilution with 30 to 300 colonies, was averaged and log transformed for statistical analysis by a 2-tailed Mann–Whitney test (significant differences identified by P values ≤0.05).
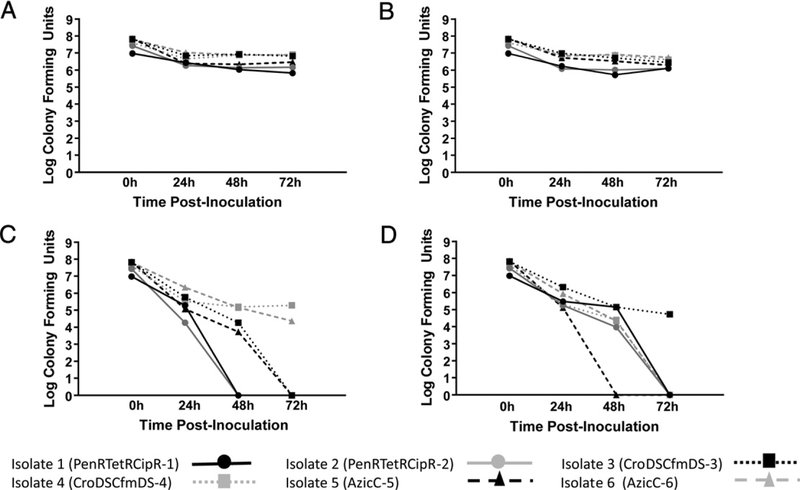
Among the 4 collection and transport platforms, the InTray GC, warmed to room temperature and incubated for 24 h at 35 °C following inoculation, yielded the best recovery of bacterial CFUs with only a one log decrease relative to the starting concentration (Fig. 1A). Using post-inoculated incubated InTray GC plates, comparable bacterial levels were measured for up to 72 hours of storage regardless of post-incubated storage temperature (Fig. 1A–B). In contrast, inoculating InTray GC plates and keeping them at room temperature or 4 °C without incubation at 35 °C resulted in a significant 2 to 4 log decrease in bacterial viability within 24 h from time of inoculation (P < 0.001) (Fig. 1C–D). At 72 h, 2 isolates were viable if the InTray GC plates were kept at room temperature (Fig. 1C) and only one viable isolate detected if kits were refrigerated after inoculation (Fig. 1D).
Fig. 1.
Detection of viable Neisseria gonorrhoeae from InTray GC systems subject to varying conditions. A, InTray GC plates incubated for 24 h at 35 °C following inoculation and held at room temperature (approximately 25 °C) for up to 72 h. Time 0 represents the colony counts performed from 100 μL aliquots of each serial dilution spread on Chocolate agar and incubated at 35 °C for 18–24 h. There were no significant (P < 0.001) differences in N. gonorrhoeae at any time interval. B, InTray GC plates incubated for 24 h at 35 °C following inoculation and held at 4 °C for up to 72 h. Time 0 represents the colony counts on the InTray GC plates 24 h after incubation. There were no significant (P < 0.001) differences in N. gonorrhoeae at any time interval. C, InTray GC plates held at room temperature for up to 72 h following inoculation. Time 0 represents the colony count of the inoculum. Significant loss of viability (P < 0.001) occurred between the 0 hour and 24 hour time points, with continued loss of viability up to 72 hours, with only 2 of 6 strains having detectable levels of organisms at 72 h. D, InTray GC plates held at 4 °C for up to 72 h following inoculation. Time 0 represents the colony count of the inoculum. Significant loss of viability (P < 0.001) occurred between the 0 hour and 24 h time points, with continued loss of viability up to 72 h, with only one of 6 strains having detectable levels of organisms at 72 h.
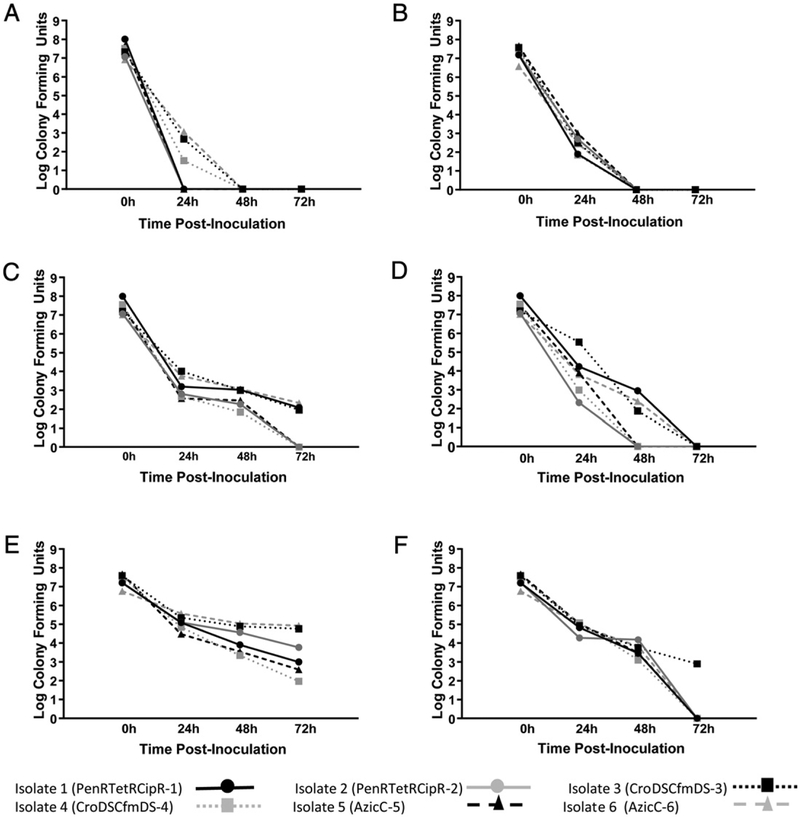
For non-nutritive transport systems, there was a large decrease in bacterial viability following 24 h (P = 0.009) and a complete loss at 48 h (P < 0.0001) when the BD CultureSwab W/Media was used to inoculate Amies medium (Fig. 2A–B). A similar but less dramatic trend was observed with the BD CultureSwab MaxV with all isolates maintaining viability at 48 h with a 5 to 6 log decrease if Amies medium was refrigerated post-inoculation (P < 0.0001; Fig. 2C–D). The Copan Eswab was slightly better than the BD CultureSwab MaxV with an approximate 4 log decrease after 24 h (P ≤ 0.004) (Fig. 2E–F), and only a modest change between 24 and 48 h (P = 0.01) at ambient temperature storage (Fig. 2F). At 72 h, all isolates remained viable at low concentrations from refrigerated media (Fig. 2E), but only one isolate remained viable if the Amies medium containing the Copan Eswab was kept at room temperature post-inoculation (Fig. 2F). There were no observed differences in bacteria viability associated with isolates with dissimilar antimicrobial susceptibility patterns.
Fig. 2.
Detection of Viable Neisseria gonorrhoeae from non-nutritive culture systems subject to varying conditions. A, N. gonorrhoeae spiked BD CultureSwab W/Media and inoculated into Amies medium which were then refrigerated at 4 °C post-inoculation. Complete loss of viability by 48 h (P b 0.0001). B, N. gonorrhoeae spiked BD CultureSwab W/Media and inoculated into Amies medium which were then stored at room, temperature (approximately 25 °C) post-inoculation. Complete loss of viability by 48 h (P < 0.0001). C, N. gonorrhoeae spiked BD CultureSwab MaxV and inoculated into Amies medium which were then refrigerated at 4 °C post-inoculation. Three of 6 strains were viable at 72 h. D, N. gonorrhoeae spiked BD CultureSwab MaxV and inoculated into Amies medium which were then stored at room, temperature (approximately 25 °C) post-inoculation. Complete loss of viability at 72 h (P < 0.0001). E, N. gonorrhoeae spiked Copan Eswab and inoculated into Amies medium which were then refrigerated at 4 °C post-inoculation. All strains had varying degrees of loss, but all with detectable levels at 72 h. F. N. gonorrhoeae spiked Copan Eswab and inoculated into Amies medium which were then stored at room temperature (approximately 25 °C) post-inoculation. Only one strain remained viable at 72 h.
Incubation of inoculated InTray GC plates at 35 °C for 24 h provided optimal recovery of N. gonorrhoeae whether the plates were kept at room temperature or refrigerated post-inoculation. Viability was maintained for up to 72 h when InTray GC plates were incubated following inoculation, which contrasted to the poor bacterial viability observed on InTray GC plates that were not incubated following inoculation. The use of the Copan Eswab yielded slightly better viability than the BD CultureSwab MaxV with Amies medium, with all isolates being recovered in low concentrations after 48 h. The performance of the BD CultureSwab was extremely poor; all isolates were unrecoverable after 48 h. The use of various clinical isolates did not affect this study’s outcome in contrast to a previous study with marked differences in the recovery of various N. gonorrhoeae auxotypes suggesting that isolate selection is important when assessing the performance of transport media (Graver & Wade, 2004).
This study clearly demonstrates that a nutritive system such as the InTray GC would be optimal to transport viable N. gonorrhoeae to the laboratory. These results support a previous study that demonstrated equivalent recovery of N. gonorrhoeae from InTray GC and MTM plates inoculated with endocervical specimens from women presenting at a sexually transmitted disease clinic (Beverly et al., 2000). Acceptability of a non-nutritive transport system was limited to Amies medium inoculated with either the Copan Eswab or BD CultureSwab MaxV. However, the large decrease in bacterial concentration after 24 h suggests that a large initial inoculum would benefit recovery. InTray GC plates may be considered for the collection and transport for viable N. gonorrhoeae isolates in settings where transit time may be greater than 24 h.
Acknowledgements
This project was 100% funded with federal funds from a federal program of $98,023. The study was supported by Cooperative Agreement # U60HM000803 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC or the Department of Health and Human Services.
The authors thank Susie Zanto and Kevin Pettus for excellent support.
Footnotes
Conflicts of interest
There were no conflicts of interest by any author. The use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References
- Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria Gonorrhoeae. Diagn Microbiol Infect Dis 2000;36:163–8. [DOI] [PubMed] [Google Scholar]
- Beverly A, Bailey-Griffin JR, Schwebke JR. InTray GC medium versus modified Thayer-Martin agar plates for diagnosis of gonorrhea from endocervical specimens. J Clin Microbiol 2000;38:3825–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae – 2014. MMWR 2014; 63(RR-2):1–19. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Gonococcal isolate surveillance project(GISP). http://www.cdc.gov/std/gisp/default.htm, 2015.
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement (M100-S25). Wayne PA USA: Clinical Laboratory Standards Institute; 2015. [Google Scholar]
- Farhat SE, Thibault M, Devlin R. Efficacy of a swab transport system in maintaining viability of Neisseria gonorrhoeae and Streptococcus pneumoniae. J Clin Microbiol 2001;39:2958–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graver MA, Wade JJ. Survival of Neisseria gonorrhoeae isolates of different auxotypes in six commercial transport systems. J Clin Microbiol 2004;42:4803–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magooa MP, Muller EE, Gumede L, Lewis DA. Determination of Neisseria gonorrhoeae susceptibility to ciprofloxacin in clinical specimens from men using a real-time PCR assay. Int J Antimicrob Agents 2013;42:63–7. [DOI] [PubMed] [Google Scholar]
- Nicol M, Whiley D, Nulsen M, Bromhead C. Direct detection of markers associated with Neisseria gonorrhoeae to ciprofloxacin antimicrobial resistance in New Zealand using residual DNA from the Cobas 4800 CT/NG NAAT assay. Sex Transm Infect 2015;91:91–3. [DOI] [PubMed] [Google Scholar]
- Peterson SW, Martin I, Demczuk W, Bharat A, Hoang L, Wylie J, et al. Molecular assay for detection of genetic markers associated with decreased susceptibility to cephalosporins in Nesseria gonorrhoeae. J Clin Microbiol 2015;53:2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ, Pandori M, Castro L, Barry P, Whittington WL, Liska S, et al. Real-time PCR assay for the detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol 2007;45:1250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014;27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn KG, Audette CD, Sebeck D, Tucker KA. Comparison of the Copan ESwab system with two Amies agar swab transport systems for maintenance of microorganism viability. J Clin Microbiol 2008;46:1655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Graver M. Refrigeration does not compromise recovery of Neisseria gonorrhoeae from charcoal transport swabs. Sex Transm Infect 2005;81:93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JJ, Graver MA. Survival of six auxotypes of Neisseria gonorrhoeae in transport media.J Clin Microbiol 2003;41:1720–1. [DOI] [PMC free article] [PubMed] [Google Scholar]