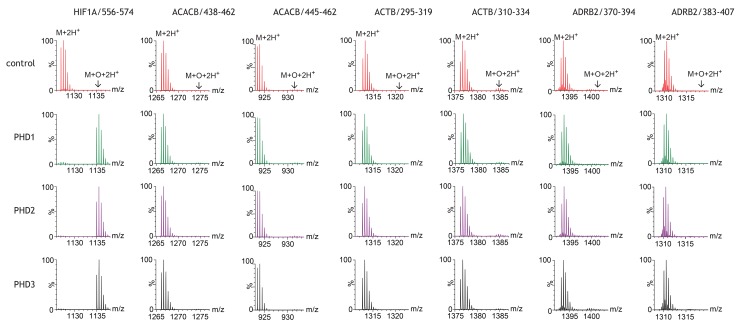
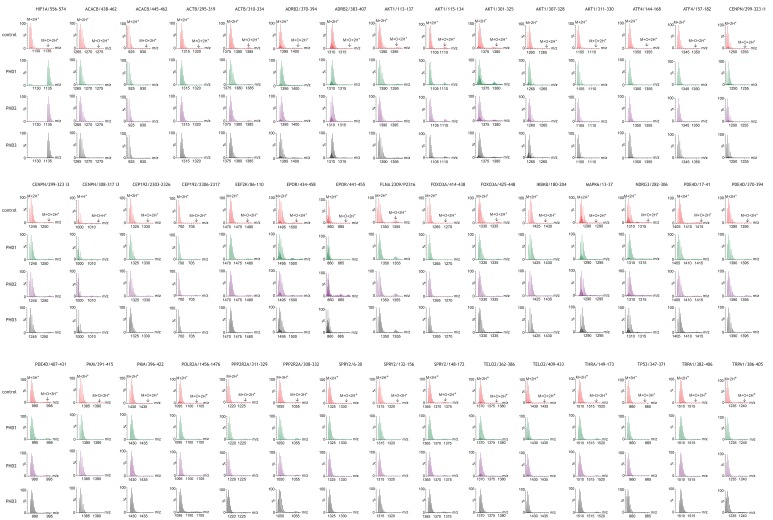
Figure 1. Assays of peptide hydroxylation.
LC-MS spectra of peptides derived from HIF-1α (left) and selected non-HIF peptidyl substrates (see Figure 1—figure supplement 1 for complete dataset) reacted with the indicated PHD isoform, or no PHD enzyme (control). In control reactions the doubly-charged (M+2H+) peptides showed the calculated mass. Following incubation with PHDs, only the doubly-charged HIF-1α peptide mass is shifted by an m/z increment of 7.997 Da (M+O+2H+) indicative of prolyl hydroxylation. No PHD-dependent mass shift was observed on any of the non-HIF substrates.