Figure 1.
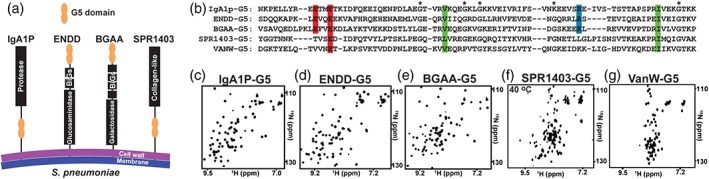
Single copy Streptococcus pneumoniae G5‐containing proteins give rise to well‐dispersed spectra. (a) Cartoon representation of the four S. pneumoniae proteins for which their G5 modules have been expressed in this study. (b) Sequence alignment of S. pneumoniae IgA1P‐G5, ENDD‐G5, BGAA‐G5, SPR1403‐G5 and Enteroccoccus faecium VanW‐G5 with conserved acidic (red), basic (blue), and hydrophobic (green) residues, and the original delineation of 5 glycine residues that are in fact not all conserved (*). 15N‐HSQC spectrum of the (c) IgA1p‐G5, (d) ENND‐G5, (e) BGAA‐G5, (f) SPR1403‐G5, and (g) VanW‐G5. Spectra were recorded at 900 MHz at 25°C, excluding SPR1403‐G5 that exhibits extreme line‐broadening at such lower temperatures (see Figure 2)
