Figure 4.
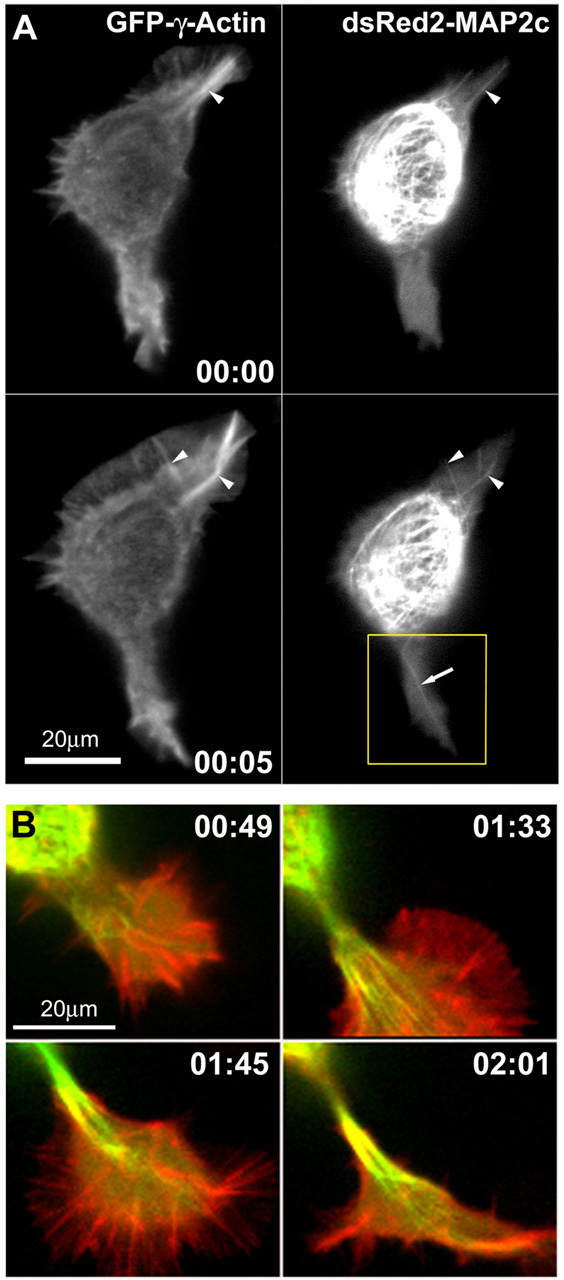
Changes in microtubules and F-actin during neurite initiation. A, MAP2c-decorated microtubules frequently align with actin cables in lamellipodia of Neuro-2a cells (also see Fig 4.mov; time points represent hours:minutes). Neuro-2a cells were cotransfected with plasmids encoding GFP-γ-actin and dsRED2-MAP2c and imaged using a spinning disk confocal microscope. Red and green fluorescence signals were acquired at intervals of 10 sec, with a time delay of 3.5 sec between the two channels. Single MAP2c-decorated microtubules (arrow) frequently invade deeply into the lamellipodia and align with actin cables (arrowheads). At time 0:05, a large actin bundle in the lamellipodium at the top right breaks, and an associated microtubule bundle appears to break in concert. The boxed region is shown in B at later time points, as this protrusion goes on to produce a neurite. B, Disordered microtubule arrays become ordered and aligned into bundles to form a neurite shaft. These panels depict four later time points in observations of the lower lamellipodium shown in A. Images are digital overlays of the GFP-actin fluorescence shown here in red and the dsRed2-MAP2c fluorescence shown here in green. Initially, the process is invaded by multiple microtubules, which are disorganized (0:44) but eventually become aligned into quasiparallel arrays (1:33). Thereafter, the microtubule array becomes quickly condensed into a compact bundle (1:45). The process elongates as microtubules continue to advance distally into the growth cone, and they become tightly packed into bundles behind the growth cone core (02:01).
