Abstract
Local protein synthesis is required for long-lasting synapse-specific plasticity in cultured Aplysia sensorimotor synapses. To identify synaptically localized mRNAs, we prepared a cDNA library from isolated sensory neurites. By sequence analysis, we estimate that the library contains 263 distinct mRNAs, with 98 of these mRNAs constituting 70% of all clones. The localized transcripts are enriched for mRNAs encoding cytoskeletal elements and components of the translational machinery. In situ hybridization confirms that the mRNAs for at least eight of these transcripts are present in distal neurites. Immunocytochemistry reveals that serotonin regulates the translation of one of the localized mRNAs, that encoding α1-tubulin. Our identification of mRNAs encoding cytoskeletal elements suggests that local protein synthesis is required for the growth of new synaptic connections associated with persistent synaptic strengthening. Our finding of mRNAs encoding components of the translational machinery suggests that local protein synthesis serves to increase the translational capacity of synapses.
Keywords: synaptic plasticity, mRNA localization, translation, synaptic tagging, Aplysia, cytoskeleton
Introduction
Long-lasting forms of learning-related synaptic plasticity require RNA and protein synthesis. We described previously a form of synapse-specific, transcription-dependent plasticity at the Aplysia sensory-to-motor connection that depends on local protein synthesis in sensory neurites (Martin et al., 1997b; Casadio et al., 1999). We further found that isolated sensory neurites, from which the cell bodies had been removed, were capable of translation and that this translation was stimulated by serotonin (5-HT) (Martin et al., 1997b). These studies indicated that mRNAs are present in the sensory neurite and that 5-HT-regulated translation of these mRNAs contributes to synapse-specific plasticity. We have now undertaken to identify the population of transcripts present in the sensory cell processes.
Several mRNAs have been detected previously in the dendrites of vertebrate neurons or the processes of invertebrate neurons (Steward and Schuman, 2001). The dendritic-neuritic localization of these mRNAs was discovered fortuitously in the course of in situ hybridization studies. Microarray studies of dendritically localized mRNAs in rodent hippocampal neurons have indicated that several hundred mRNAs are present (Eberwine et al., 2002). By mechanically separating and removing cell bodies from the neurites of cultured Aplysia sensory neurons, we generated a pure preparation of sensory neuronal processes. We used this preparation to create a cDNA library as a means of identifying, in an unbiased manner, the population of mRNAs present in unstimulated sensory neuron processes. Sequence analysis indicates that the Aplysia sensory neuron process library contains 263 distinct mRNA transcripts, of which 98 comprise 69% of all clones sequenced from the peripheral library. We studied a subset of these transcripts in more detail and have confirmed by in situ hybridization that the mRNAs are localized to the distal neurites of the Aplysia sensory neurons. These transcripts are enriched for mRNAs encoding cytoskeletal elements and for components of the translational machinery.
The translational components include mRNAs encoding the cytoplasmic polyadenylation element binding protein (CPEB), elongation factor 1α (EF1α), and several mRNAs for ribosomal proteins, including two proteins important for bridging the 40 S ribosomal subunit to the 60 S subunit. This finding suggests that one function of local protein synthesis is to ensure, in a synapse-specific manner, the translational competence of local ribosomes. One of the cytoskeletal mRNAs identified from our library encodes the Aplysia homolog of α1-tubulin, and 5-HT application to isolated sensory neurites increases its translation.
Our results indicate that there is a complex repertoire of mRNAs present at the synapse and that 5-HT is capable of regulating the translation of at least some of these mRNAs. Our identification of mRNAs encoding cytoskeletal elements suggests that local protein synthesis is required for the growth of new synaptic connections associated with persistent synaptic strengthening. Our finding of mRNAs encoding components of the translational machinery suggests that local protein synthesis serves to increase the translational capacity of synapses. We hypothesize that this provides a translational sink that “tags” a synapse for persistent facilitation by translating locally stored mRNAs and capturing mRNAs exported from the nucleus to the neurites after 5-HT-induced transcription.
Materials and Methods
Aplysia cell culture. Culture dishes and medium were prepared as described previously (Montarolo et al., 1986). Bifurcated sensory neuron-motor neuron cultures were prepared as described by Martin et al. (1997b). Details of culture methods can be found at the following website: http://www.gonda.ucla.edu/researchlabs/martin/Protocalls.htm. Cultures were maintained for 5 d in an 18°C incubator. All animals were purchased from either Alacrity (Redondo Beach, CA) or from the University of Miami National Institutes of Health Aplysia resource facility (Miami, FL).
Aplysia sensory neuronal process cDNA library construction and analysis. Four hundred Aplysia sensory neurons (100 neurons each in four dishes) were cultured in medium containing filtered hemolymph, and the hemolymph concentration was reduced from 50% on day 1, to 20% on day 3, 10% on day 4, and 0% on day 5. On day 5, the cell body of each neuron was removed by cutting at a distance of ∼50 μm distal to the cell soma-initial axon junction using a glass microelectrode, and the cell bodies were removed by aspiration. RNA was extracted from the isolated neurites using Trizol (Invitrogen, Gaithersburg, MD) and treated with RNase-free DNase. This RNA was used as the starting material for cDNA library construction following the method of Brady and Iscove (1993) (see also Dulac and Axel, 1995). Briefly, the RNA was reverse transcribed for 10 min using low concentrations of oligo-dT and of nucleotides to limit the cDNA length to between 500 and 1000 bp. The first-strand cDNA was then tailed with poly(A+) using terminal transferase. This cDNA was then PCR amplified (50 cycles) using primers containing oligo-dT and an EcoRI site. After EcoRI digestion, the resulting cDNA was ligated into lambda ZAPII (Stratagene, La Jolla, CA) and packaged in GIGApack gold (Stratagene). The unamplified library contained 15,600 clones with inserts. We performed mass excision of the Aplysia Sensory Neuron Process (ASNP) library phagemids following the protocol of the manufacturer, plated the product on LB-ampicillin plates containing isopropyl-β-d-thiogalactopyranoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), and selected all white colonies for sequencing by the Columbia University Genome Facility (New York, NY) or by the University of California at Davis sequencing facility (Davis, CA). Sequence analysis was performed using VectorNTI Suite 6.0 and NCBI BLAST. Clones were accepted as identified when they showed strongly significant BlastN or BlastX alignments (Altschul et al., 1997), which did not depend on repetitive sequences.
Representation of clones was determined by screening 100,000-500,000 phage each from the ASNP library and from a full-length cDNA phage library prepared from Aplysia sensory clusters (kindly provided by D. Bartsch, Central Institute of Mental Health, Mannheim, Germany). Inserts from randomly selected ASNP clones were nick translated and hybridized to filters lifted from library plates in 50% formamide, 6× SSC, 0.5% SDS, 5× Denhardt's solution, and 100 μg/ml sheared salmon sperm DNA for 24 hr and were then washed twice at high stringency in 0.2× SSC and 0.2% SDS at 65°C for 20 min each before exposure to x-ray film. Representation was expressed as a percentage of positive clones compared with the total number of phage with inserts. We determined the percentage of phage with inserts in each library by blue and white selection on IPTG/X-Gal plates. Full-length sequences and sequences containing significant amounts of coding sequence have been submitted to the GenBank database. Accession numbers are as follows: apα1-tubulin, AF481055; apα2-tubulin, AF481056; β-thymosin, AF481063; L8, AF481057; L18, AF481060; L31, AF481064; S15, AF481061; S16, AF481058; L40-ubiquitin, AF481059; S27-ubiquitin, AF481062; S29, AF486840; S19, AF486814; L37, AF468842; and L36, AF486843.
In situ hybridization. Digoxigenin sense and antisense riboprobes were made using digoxigenin-UTP (Roche Products, Hertforshire, UK) and the MaxiScript transcription kit (Ambion, Austin, TX) according to instructions of the manufacturer. Riboprobes ranged in length from 400 to 600 bp. The labeling efficiency of the transcription was determined by dot-blotting using a control digoxigenin-labeled RNA, according to the instructions of the manufacturer (Roche Products); the concentration of each probe was determined by spotting dilutions of the probe on an ethidium bromide-containing agar plate and comparing the signal to that produced by dilutions of a digoxigenin-labeled control RNA (Roche Products).
To prepare cryostat sections for in situ hybridization, ganglia were dissected from 80-100 gm animals, fixed in 4% paraformaldehyde-30% sucrose in PBS for 1.5 hr at room temperature, and frozen in OCT. Twenty-micrometer-thick sections were cut on a Mikrom cyrostat, air dried for 30-60 min, and then postfixed for 10 min in 4% paraformaldehyde. To prepare cultured neurons for in situ hybridization, cells were fixed in 4% paraformaldehyde-30% sucrose in PBS for 10 min at room temperature and then washed in DEPC-treated PBS. Cryostat sections and cultured neurons were then processed for in situ hybridization using the same methods. Samples were carbethoxylated by incubation with 0.1% active DEPC for two times at 15 min each at room temperature, and samples were then equilibrated in DEPC-treated 5× SSC for 15 min and prehybidized for 2 hr at 58°C in 50% formamide, 5× SSC, 40 μg/ml salmon sperm DNA, and 2% blocking reagent (Roche Products). They were then hybridized for 18-48 hr at 58°C (or as otherwise determined for the melting temperature of each riboprobe), with 400 ng/ml labeled probe, 50% formamide, 5× SSC, 40 μg/ml salmon sperm DNA, and 2% blocking reagent (Roche Products). They were then washed for 30 min in 2× SSC at room temperature, for 1 hr in 2× SSC at 65°C, and for 1 hr in 0.1× SSC at 65°C. After a 5 min equilibration in 100 mm Tris, pH 7.5, and 150 mm NaCl (Tris/NaCl), samples were incubated with anti- digoxigenin antibody coupled to alkaline phosphatases (Roche Products) diluted 1:5000 in Tris/NaCl buffer containing 0.5% blocking reagent overnight at 4°C. The next day, they were washed twice for 15 min each in Tris/NaCl buffer and equilibrated for 5 min in 100 mm Tris, pH 9.5, 100 mm NaCl, and 50 mm Mg2Cl (AP buffer). They were then stained in AP buffer containing nitroblue-tetrazolium-chloride and 5-bromo-4-chloro-3-indolyl-phosphate for anywhere from 1 to 48 hr; the reaction was monitored, and both sense and antisense samples were stopped at the same time by washing with water for 15 min and then incubating in 95% ethanol for 1 hr. Stained samples were visualized on a Zeiss (Oberkochen, Germany) Axiovert 135 microscope using 10× dry, 40× oil, or 63× oil objectives. Most images were acquired using a Sony (Tokyo, Japan) digital still camera; however, some images were acquired using a Nikon (Tokyo, Japan) 35 mm SLR camera.
Immunoblotting and immunocytochemisty. Immunoblotting of Aplysia CNS was done as described previously (Michael et al., 1998), using a monoclonal anti-α-tubulin antibody purchased from Calbiochem (San Diego, CA). Immunocytochemistry with anti-α-tubulin antibodies was done as described previously (Martin et al., 1997a). Each experiment was performed by one individual and imaged by a different individual (blind to experimental conditions) on a Zeiss Axiovert 135 microscope using 10× dry and 63× oil objectives. Images were acquired using a Hamamatsu (Bridgewater, NJ) Orca CCD camera driven by Universal Imaging Corporation (West Chester, PA) Metamorph software. All images were taken using 30% of the 100 W mercury lamp intensity and were taken using the same exposure times within a single experiment. Pixel intensity in neurites was determined using Universal Imaging Corporation Metamorph software by thresholding above background, creating an area of interest around the thresholded objects (the neurites), and measuring the average pixel intensity within that area (the neurite). In some of the initial experiments, images were acquired using Zeiss Axiovision software, and pixel intensity was measured using regions of interest in distal neurites with the Scion Image (NIH) software. To determine the effect of local stimulation on immunoreactivity, images were taken during the perfusion, and a region of 25 μm adjacent to the perfusion electrode was selected for measurement. For isolated sensory neurons, a region in an unperfused neurite, at the same distance from the cell body, was used as the untreated region. For bifurcated cultures, the region (25 μm) of the sensory neurite contacting the motor neuron at the untreated branch was used. To ensure that we were measuring immunoreactivity in the sensory and not the motor neurite, measurements were made in the sensory neurite just before it contacted the motor neuron. All measurements were logged into a Microsoft (Seattle, WA) Excel spreadsheet and then transferred into Prism software (GraphPad Software, San Diego, CA) for statistical analysis. Comparisons were made between multiple experimental groups by ANOVA, followed by a Newman-Keuls multiple comparison test. When only two groups were tested, an unpaired Student's t test was used.
Pharmacological treatment of cultures. Bath application of five pulses of 5-HT to cultured Aplysia sensory neurons was done as described previously (Montarolo et al., 1986). Briefly, five 5 min applications of 5-HT (10 μm) were applied at 20 min intervals. Cells were fixed 30 min after the final 5-HT application. To test the role of protein synthesis in the 5-HT-induced increase in α-tubulin immunoreactivity, intact Aplysia sensory neurons or isolated sensory cell neurites (immediately after removal of the cell bodies) were treated with emetine (100 μm) for 30 min previous, during, and for 30 min after application of five pulses of 5-HT. Local stimulation with 5-HT was done as described previously (Martin et al., 1997).
Results
Preparation of a cDNA library from pure Aplysia sensory neuronal processes
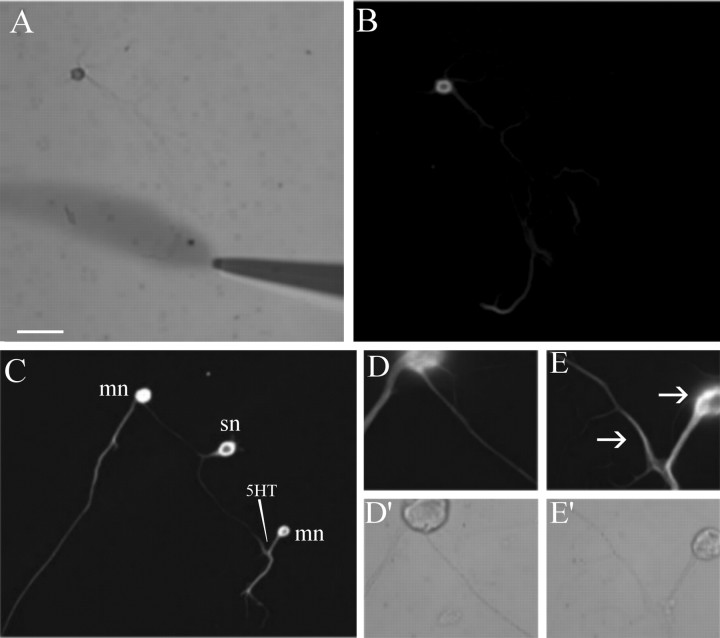
Four hundred sensory neurons were plated on poly-l-lysine-coated dishes. After 5 d in culture, the sensory cell bodies and initial axon segments were removed using a sharp electrode, leaving a preparation of pure sensory neuronal processes (Fig. 1A-C). Because of the way in which Aplysia neurons are cultured, the only glia that are present are attached to the neuronal cell body. Thus, after removal of the cell body, no glia remained in the culture dish. We used propidium iodide staining to show that no DNA (and hence no cell bodies) remained in the culture dish (Fig. 1d,e).
Figure 1.
A pure preparation of isolated Aplysia sensory neurons as the starting material for cDNA library synthesis. Aplysia sensory cells were cultured for 5 d (A), and the cell bodies and ∼50 μm of the proximal axon segment were transected from the distal neurites using a sharp electrode (B). The cell bodies-proximal axon segments were then removed by aspiration, leaving a preparation of pure sensory neurites (C). Isolated sensory neurites were free of glia, as shown by propidium iodide (red) staining of sensory cell cultures before (D) and after (E) removal of cell bodies, which shows that there is no DNA left after removal of the sensory cell somata. Total RNA was prepared from sensory cell neurites and used to prepare cDNA by RT-PCR. The amplified cDNA was shown to contain mRNAs of neuritic but not glial origin by Southern blotting using a probe encoding sensorin, an mRNA known to be present in sensory cell processes, and a probe encoding a glial antigen (ag). Scale bar, 100 μm.
Total RNA was isolated from the neurites and used as the starting material for the preparation of a cDNA library using time-limited, oligo-dT primed reverse transcription (RT)-PCR (Brady and Iscove, 1993). The rationale for this approach is that it maintains a relatively accurate representation of mRNA abundance even when the starting material is extremely limited. After PCR, the amplified cDNA was analyzed by Southern blot to confirm its neuritic origin. We hybridized our Southern blot with a positive control probe encoding sensorin, an mRNA known to be present in the processes of Aplysia sensory neurons (Brunet et al., 1991), and, as a negative control, a glial-specific probe (Lockhart et al., 1996). As shown in Figure 1f, the amplified cDNA contains sensorin but no glial specific antigen.
This amplified cDNA was used to make a phagemid library. We named this cDNA library the ASNP library. We sequenced 668 clones from the ASNP library (4.3% of all clones in the library). Of these, 533 encoded mRNAs (of the remaining clones, 70 encoded rRNA, tRNA, or scRNA, and 65 contained sequences too short to identify). Analysis of the 533 mRNAs revealed that they comprised 263 unique transcripts. Of these, 98 transcripts accounted for 69% of the clones in the library (368 of the 533 mRNAs).
Identification of cDNAs in the ASNP library
As described above, we chose to use time-limited RT-PCR to maintain a relatively accurate representation of mRNAs in the neuronal process preparation. As a result of this strategy, the clones in the ASNP library were only 100-800 bp long (with a mode of 300), and, because the reverse transcription was primed using oligo-dT, the clones represented 3′ expressed sequence tags. These were primarily restricted to the 3′ untranslated region of mRNAs. Because there is very little conservation of sequences in untranslated regions between species and because the Aplysia database is limited, most of the sequences we obtained did not show homology to any sequences in existing databases. Thus, to determine the identity of the transcripts in the ASNP library, we undertook a second round of cloning using sequences from the ASNP library to screen a full-length library made from sensory cell clusters (Bartsch et al., 1998). As a result of full-length cloning and because some ASNP clones contained sufficient sequence from the coding region to detect homology with sequences from other species and because some Aplysia cDNAs were present in the sequence database, we determined the identity of 18 clones in the ASNP library (Table 1). These include an isoform of α-tubulin most similar to α1-tubulin, the actin binding protein β-thymosin, the peptide neurotransmitter sensorin, an Aplysia fasciclin-like protein, and 14 molecules involved in translation, including the translation elongation factor EF1α, CPEB, the ribosomal proteins S6, S15, S16, S19, S29, L8, L11, L18, L31, L36, and L37, and cDNAs encoding L40 and ubiquitin and S27 and ubiquitin as translational fusions.
Table 1.
Clones identified in the ASNP library
|
Class of molecules |
Identity of clone |
Frequency (% of sequenced clones) |
|---|---|---|
| Cytoskeletal | Tα1-tubulin | 15% |
| β-Thymosin | 2.2% | |
| Translation | EF1α | 0.4% |
| CPEB | 0.2% | |
| Ubiquitin-L27 | 0.2% | |
| Ubiquitin-S40 | 0.2% | |
| L8 | 0.2% | |
| L18 | 0.4% | |
| L31 | 0.2% | |
| S6 | 0.2% | |
| S15 | 0.9% | |
| S16 | 0.2% | |
| Transmitter release | Sensorin | 1% |
| Adhesion
|
Fasciclin-like
|
0.2%
|
Many mRNAs are more highly represented in the ASNP library than in a sensory cell cluster library
It is conceivable that mRNAs reach neuronal processes by simple diffusion from the cell body. Were this true, the most abundant mRNAs from the cell body should be most abundant in the ASNP library. To address this possibility, we hybridized specific ASNP clones to both the ASNP library and a cDNA library made from clusters of sensory neuron cell bodies (Bartsch et al., 1998) to determine whether there were any clones that had a greater representation in the ASNP library than in the sensory cell body cluster library. As shown in Table 2, although some clones, such as that encoding sensorin, are more highly represented in the sensory cluster library, many other clones (including α-tubulin, β-thymosin, fasciclin-like protein, and ribosomal protein L18) are more highly represented in the ASNP library. It is important to note that this does not necessarily mean that these cDNAs are more abundant in absolute terms, but rather that they constitute a larger percentage of the total population of mRNAs in the process than of the total population of mRNAs in the cell body. These results do, however, suggest that the mRNAs present in the sensory cell process are transported by an active transport mechanism rather than by passive diffusion out of the cell body.
Table 2.
Representation of clones in the ASNP library compared with representation in a sensory cell cluster library
|
Clone |
ASNP library |
Sensory cluster library |
Enrichment |
|---|---|---|---|
| ASNP 161 | 0.02% | <0.0001% | >200× |
| Fasciclin-like protein | 0.01% | <0.0001% | >100× |
| β-Thymosin | 0.4% | 0.004% | 100× |
| ASNP 6 | 0.1% | <0.0002% | >50× |
| ASNP 20 | 1.9% | 0.04% | 47.5× |
| ASNP 53 | 1.8% | 0.04% | 45× |
| ASNP 50 | 2.2% | 0.06% | 36.7× |
| ASNP 40 | 0.05% | <0.002% | >25× |
| Contig 86 | 0.05% | <0.002% | >25× |
| α1-Tubulin | 6.2% | 0.4% | 15.5× |
| L18 | 0.04% | 0.003% | 13.3× |
| ASNP 82 | 1.4% | 0.17% | 8× |
| Sensorin | 0.7% | 2.0% | 0.33× |
| α2-Tubulin
|
<0.002%
|
0.04%
|
.005×
|
Localization of ASNP clones to sensory neurites by in situ hybridization
To confirm that the transcripts present in the ASNP library are in fact present in neuronal processes, we performed in situ hybridization in cultured Aplysia neurons. As shown in Figure 2, mRNAs encoding β-thymosin and ribosomal proteins S6, L18, and L8 are present not only in the cell bodies but also in the neurites of cultured sensory neurons (Fig. 2, top four panels on left). There is no signal when sense riboprobes are used (Fig. 2, top four panels on right). As a corollary, not all mRNAs are localized to the distal neurites in cultured Aplysia sensory neurons. Thus, as shown in Figure 2 (bottom panel), the mRNA encoding Aplysia CCAAT/enhancer binding protein (C/EBP), a transcription factor that is robustly induced in sensory neurons during 5-HT application, is restricted in its localization to the cell body of sensory neurons. In situ hybridization was also performed for sensorin and two of the unidentified clones, and these transcripts were also found to be present in distal processes (data not shown).
Figure 2.
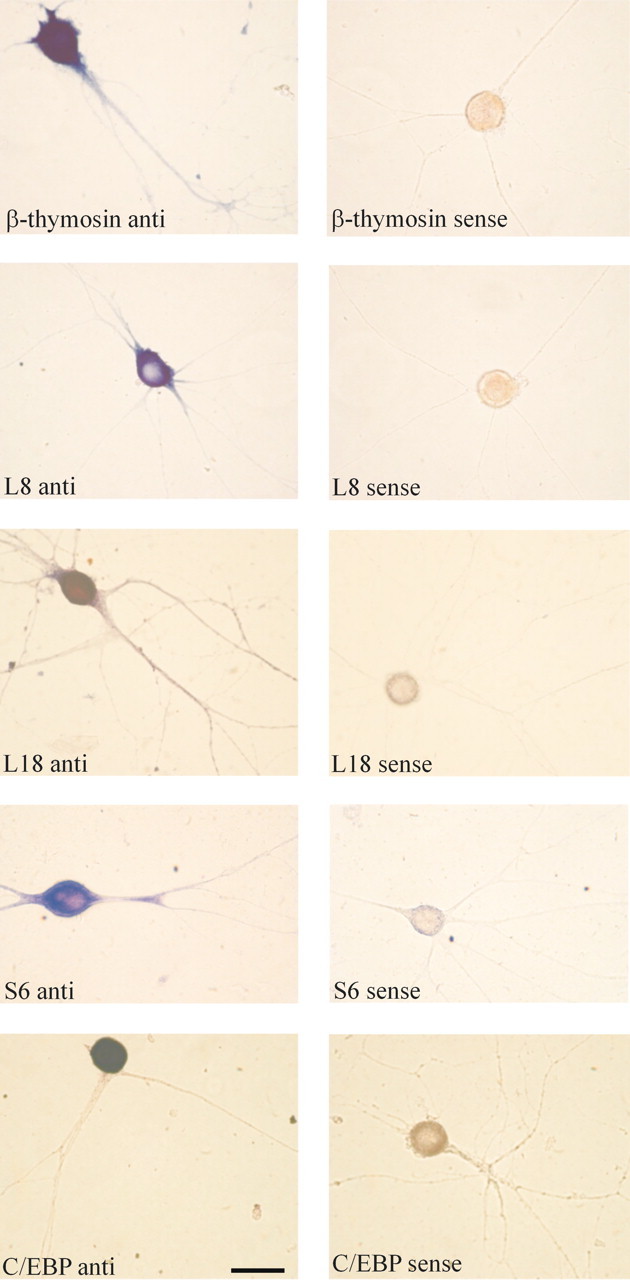
mRNAs encoding the actin-binding protein β-thymosin and ribosomal proteins L8, L18, and S6 are present in distal sensory neurites. Aplysia sensory neurons were cultured for 5 d, fixed, and hybridized with antisense (left) and sense (right) riboprobes for the actin binding protein β-thymosin, the ribosomal protein L8, the ribosomal protein L18, and the ribosomal protein S6. All four mRNAs are present not only in the sensory cell bodies but also in the distal neurites. To demonstrate that not all mRNAs are present in the neurites of Aplysia neurons, we gave five applications of 5-HT to cultured sensory neurons, which results in a robust induction of the transcription factor C/EBP (Alberini et al., 1994), fixed the cells, and hybridized with antisense (left) and sense (right) riboprobes for C/EBP. Whereas the sensory cell body has intense staining for C/EBP mRNA, there is no staining in the neurites. Scale bar, 50 μm.
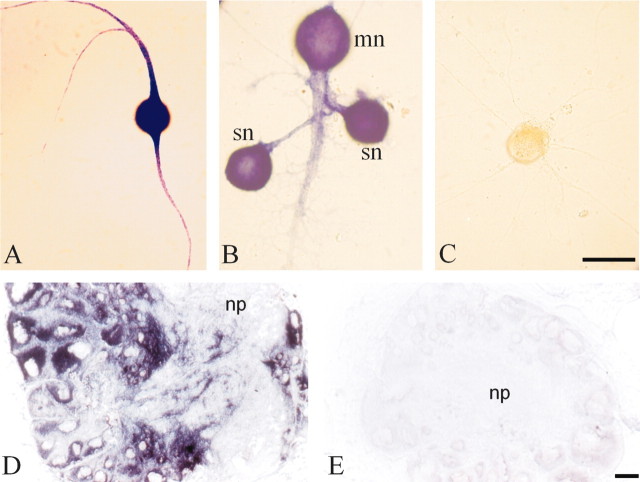
The most abundant clone we obtained encoded an isoform of α-tubulin most similar to brain-specific α1-tubulin (Hall and Cowan, 1985; Miller et al., 1987). We call this α-tubulin isoform apα1-tubulin. In the course of its full-length cloning, we identified a second isoform of α-tubulin, which we term apα2-tubulin. These two isoforms of α-tubulin are 68% identical at the nucleotide level, 84% identical at the nucleotide level within the coding region, and 96% identical at the amino acid level. Using probes specific to each isoform, we found that apα1-tubulin represented 6.2% of the clones in the ASNP library but only 0.4% of the clones in a sensory cell cluster library (Table 2). In contrast, we were unable to detect any apα2-tubulin clones after screening 50,000 clones in the ASNP library, indicating that the representation of the apα1 isoform was <0.002%, whereas it was present at 0.04% of the sensory cell cluster library (Table 2). In situ hybridization confirmed that apα1-tubulin was present not only in the cell bodies but also in the distal neurites of cultured Aplysia sensory neurons (Fig. 3A,B) and in the distal neuronal processes of cultured Aplysia motor neurons (Fig. 3A). We also detected abundant α1-tubulin mRNA in the neuropil of dissected adult Aplysia CNS, indicating that its neuritic localization occurs in adult CNS, as well as in cultured neurons (Fig. 3D).
Figure 3.
mRNA encoding α 1-tubulin is present in distal sensory and motor neurites and in neuropil of dissected CNS. Sensory neurons were cultured for 5 d in isolation (A, C) or in synaptic contact with the motor neuron (B), fixed, and hybridized with antisense (A, B) and sense (C) riboprobes to Aplysia α1-tubulin. α1-Tubulin mRNA is present not only in the cell bodies of both sensory (sn) and motor (mn) neurons but also in the distal neurites of both cell types. Scale bar (in C): A-C, 50 μm. mRNA encoding α1-tubulin is also present in the neuropil in adult Aplysia CNS. Cryostat sections of pedal ganglia were hybridized with antisense (D) and sense (E) riboprobes for α1-tubulin. mRNA is present in cell bodies, located on the periphery of the ganglia, and in the processes that project into the neuropil (np) in the center of the ganglia. Scale bar (in E): D, E, 50 μm.
5-HT induces translation of α-tubulin in distal sensory neurites
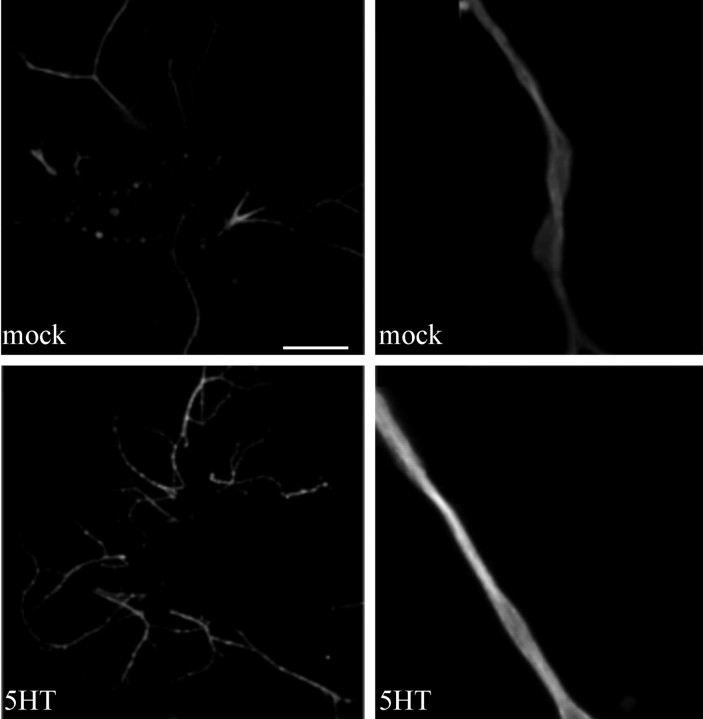
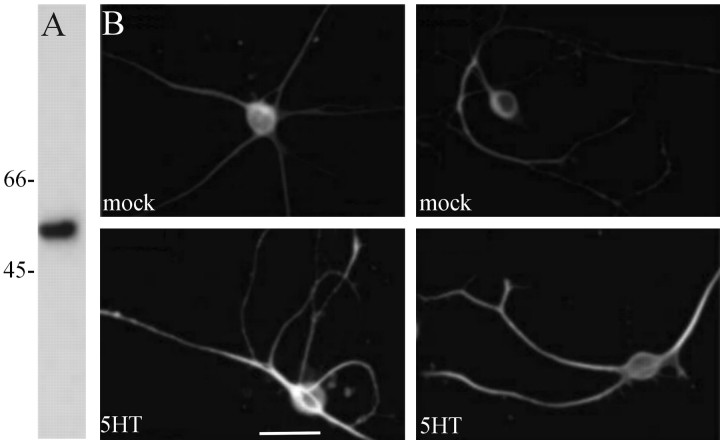
We showed previously that application of 5-HT to the neurites of sensory neurons increases translation, as detected by radioactive methionine incorporation (Martin et al., 1997b; Casadio et al., 1999). To determine whether the translation of an endogenous mRNA localized in distal processes, that encoding apα1-tubulin, is regulated by 5-HT, we used a monoclonal antibody that recognizes α-tubulin in Aplysia to quantify the amount of α-tubulin in cultured Aplysia neurons before and after exposure to 5-HT. As shown in Figure 5, the monoclonal antibody against α-tubulin recognized a band of ∼55 kDa in an immunoblot of Aplysia CNS. Staining of fixed Aplysia sensory neurons in culture with this antibody shows that α-tubulin immunoreactivity is present throughout the cell body and neurites, with the highest levels of immunoreactivity present in the neurites. Five pulses of 5-HT, sufficient to produce long-term facilitation of Aplysia sensorimotor synapses, produced an increase in α-tubulin immunoreactivity in distal neuronal processes (Fig. 4B). Quantification of the effect of five pulses of 5-HT revealed a 1.5-fold increase in α-tubulin immunoreactivity compared with mock-treated neurons (mean pixel intensity in neurites of mock-treated cells, 127 ± 4; n = 20 cells, 95 neurites; mean pixel intensity in neurites of 5-HT-treated cells, 184 ± 5; n = 19 cells, 78 neurites; p < 0.01; unpaired Student's t test). To rule out the possibility that the increase detected in the process resulted from α-tubulin that was synthesized in the cell body and transported into the process, we used a sharp electrode to remove the cell bodies and the proximal 50-100 μm of sensory cell neurons and applied 5-HT to the remaining sensory cell neurites. Five applications of 5-HT produced an increase in α-tubulin immunoreactivity in the sensory cell neurites (Fig. 5). Quantification of this effect revealed a 1.3-fold increase in α-tubulin immunoreactivity in isolated neurites with 5-HT compared with mock-treated neurites (mean pixel intensity in mock-treated neurites, 146 ± 4; n = 23 cells, 90 neurites; mean pixel intensity in 5-HT-treated neurites, 190 ± 3; n = 22 cells, 83 neurites; p < 0.01; unpaired Student's t test). This finding indicates that the modulatory neurotransmitter 5-HT is capable of regulating the local translation of α-tubulin in sensory neurites.
Figure 5.
5-HT induces the translation of α-tubulin in isolated sensory neurites. Sensory cells were cultured for 5 d, at which time the cell bodies and 50-100 μm of the proximal axon segments were removed using a sharp microelectrode. The isolated sensory neurites were treated with five pulses of 5-HT (5HT) or with medium alone (mock), fixed, and immunostained with the monoclonal anti-α-tubulin antibody. 5-HT treatment produced an increase in α-tubulin immunoreactivity in the isolated sensory neurites. Note that there appear to be hotspots of α-tubulin immunoreactivity in the 5-HT-treated neurites. Scale bar: two left panels, 50 μm; two right panels, 12.5 μm.
Figure 4.
5-HT induces the translation of α-tubulin in sensory neurons. An anti-α-tubulin antibody recognizes a single band of 55 kDa in Aplysia CNS by Western blotting (A). Sensory cells were cultured for 5 d and then treated with either five pulses of 5-HT (5HT) or mock-treated with medium alone (mock), fixed, and immunostained with the monoclonal anti-α-tubulin antibody (B). Scale bar, 50 μm.
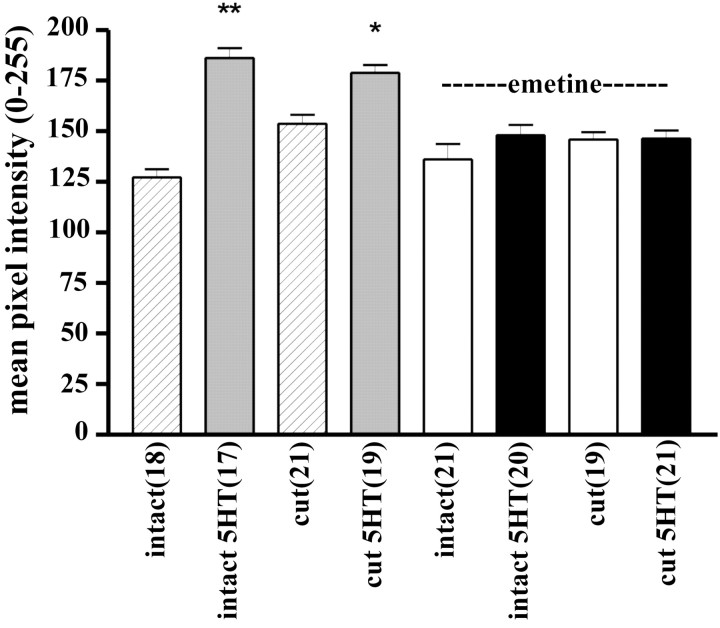
The increase in α-tubulin immunoreactivity might have resulted from an increase in tubulin polymerization. To address this possibility, we performed the experiments in the presence of a protein synthesis inhibitor, emetine. As shown in Figure 6, application of emetine completely prevented the 5-HT-induced increase in α-tubulin immunoreactivity (mean pixel intensity in emetine-treated neurites of intact neurons, 136 ± 8 in the absence of 5-HT and 148 ± 5 in the presence of 5-HT; p > 0.05; mean α-tubulin immunoreactivity in emetine-treated transected neurites in the absence of 5-HT, 145 ± 4 and in the presence of 5-HT, 146 ± 4; p > 0.05). This strongly suggests that the increase in immunoreactivity resulted from translation and not from tubulin polymerization. Interestingly, α-tubulin immunoreactivity was significantly (1.2-fold; p < 0.01) higher in the transected neurites than in the distal neurites of intact neurons, indicating that injury itself stimulated translation of α-tubulin. No such injury-induced increase in α-tubulin immunoreactivity occurred in the presence of emetine.
Figure 6.
The 5-HT-induced increase in α-tubulin in isolated sensory neurites is blocked by a protein synthesis inhibitor. α-Tubulin immunoreactivity was measured in neurites of intact neurons (intact) and in isolated sensory neurites (cut). Sensory cells were cultured for 5 d. To prepare isolated neurites, cell bodies and proximal axon segments were removed using a sharp microelectrode. Intact neurons or isolated neurites were incubated in either regular culture medium (striped and gray bars) or in medium containing emetine (100 μm) (white and black bars) for 30 min before, during, and 30 min after application of five pulses of 5-HT (5HT), fixed, and immunostained with the monoclonal anti-α-tubulin antibody. The 5-HT-induced increase in α-tubulin immunoreactivity in isolated sensory neurites was blocked by treatment with the general protein synthesis inhibitor emetine, indicating that the increase was attributable to translation. *p < 0.05; **p < 0.01; ANOVA and Newman-Keuls post hoc test.
5-HT increases the translation of α-tubulin in a spatially restricted manner
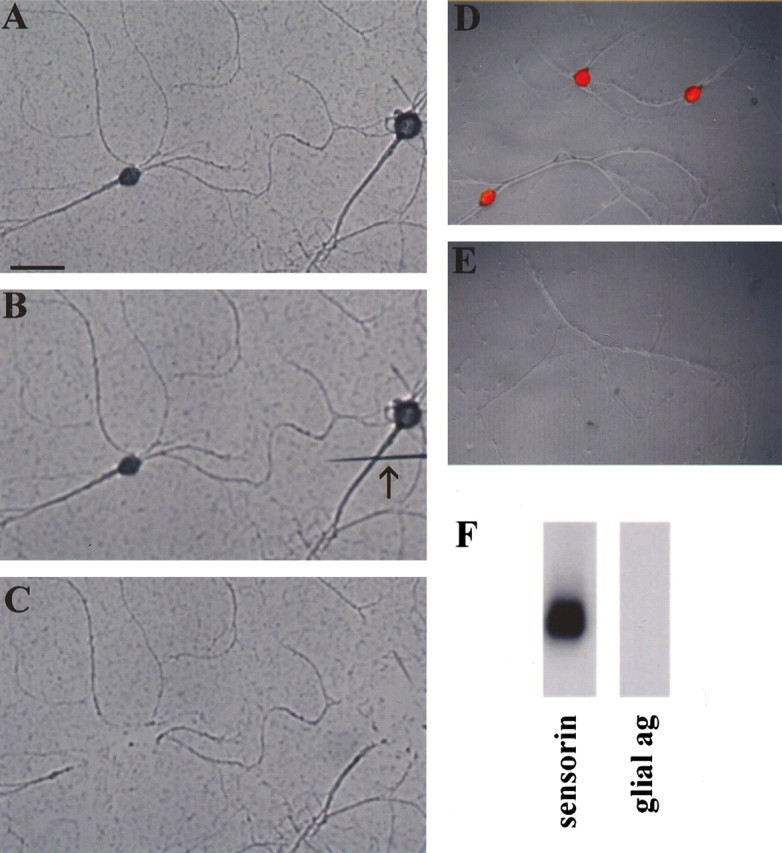
To determine whether translation of α-tubulin can occur in a spatially restricted manner, we used a perfusion microelectrode to deliver 5-HT to distal neurites of sensory cells in culture and to the connections made onto one motor neuron of a bifurcated sensory neuron in culture. As shown in Figure 7, this resulted in a localized increase in α-tubulin immunoreactivity in sensory cells (Fig. 7B) and in an increase in α-tubulin immunoreactivity at the treated but not at the untreated connection of sensorimotor synapses (Fig. 7C-E), indicating that local application of 5-HT can produce a spatially restricted increase in α-tubulin. Quantification of these results revealed that, in isolated sensory neurons, local perfusion of five pulses of 5-HT produced a 1.6-fold increase in α-tubulin immunoreactivity (mean pixel intensity in the 25 μm area adjacent to the perfusion electrode, 140 ± 13; n = 5; mean pixel intensity in a 25 μm area in a neurite not contacted by the perfusate, 87 ± 12; n = 5; p < 0.05; unpaired Student's t test). In bifurcated cultures, local perfusion of five pulses of 5-HT to one branch produced a 1.5-fold increase in α-tubulin immunoreactivity at that branch (mean pixel intensity in a 25 μm area adjacent to the perfusion electrode, 120 ± 12; n = 7; mean pixel intensity in a 25 μm area at the opposite branch, 81 ± 7; n = 7; p < 0.05; unpaired Student's t test).
Figure 7.
Local application of 5-HT produces a local increase in α-tubulin immunoreactivity. Aplysia sensory neurons were cultured for 5 d, and five pulses of 5-HT were applied locally to a distal neurite (differential interference contrast image; A). Fifteen minutes after the last pulse of 5-HT, the cells were fixed and stained with anti-α-tubulin antibodies (B). A bifurcated Aplysia sensory neuron (sn) making contact with two spatially separated LFS motor neurons (lfs) was cultured for 5 d, and five pulses of 5-HT were applied to the connection made onto one motor neuron, such that neither the sensory cell body nor the connections made onto the other motor neuron were exposed to the 5-HT (C). Increased α-tubulin immunoreactivity was detected at the connection made onto the treated (E) motor neuron compared with the untreated (D) connection; differential interference contrast images in D′, E′). Arrows point to increased immunoreactivity in distal sensory neurite and in sensory neuron varicosity forming on LFS motor neuron cell body. Scale bar (in A): A-C, 100 μm; D, E, 40 μm.
Discussion
We showed previously that mRNAs are constitutively present at the synapse in unstimulated neurons and that 5-HT-induced translation of these mRNAs is required for long-lasting, synapse-specific facilitation of sensorimotor synapses (Martin et al., 1997b; Casadio et al., 1999). Because we constructed a library from unstimulated sensory neurites, the transcripts we identify are likely to include those mRNAs whose translation is directly regulated by 5-HT and whose translation, at the time of 5-HT stimulation, is required for the establishment and maintenance of long-lasting facilitation.
Analysis of the ASNP library indicates that it contains 263 mRNA species, with 98 of these mRNAs constituting the majority of transcripts in the library. Although a select fraction of the total number of transcripts in the cell, this is a large enough number of mRNAs to provide rich complexity to the ability of the synapse to regulate its macromolecular composition. Consistent with our findings, Eberwine and colleagues have estimated that hippocampal dendrites contain ∼400 distinct mRNAs (Eberwine et al., 2002), and Kaplan and colleagues have estimated that squid giant axon contains ∼150 distinct mRNAs (Gioio et al., 2001).
The neuronal process-specific cDNA library contains mRNAs encoding cytoskeletal proteins
The lack of an adequate sequence database in Aplysia has prevented us from identifying immediately all of the clones in our library. From the 18 we identified, we can, however, draw some preliminary conclusions. Two of the mRNAs encode cytoskeletal molecules, α1-tubulin and β-thymosin. Long-term facilitation of Aplysia sensorimotor neurons involves the growth of new synaptic connections (Bailey and Chen, 1983, 1988; Glanzman et al., 1990), and it is likely that this growth requires both microtubule and microfilament networks. In fact, both α-tubulin and β-thymosin have been shown to be involved in neuronal outgrowth. The ASNP library also contains transcripts encoding a fasciclin-like protein, a member of an adhesion molecule family that has been shown to be involved in neuronal outgrowth (Martin and Kandel, 1996).
Expression of brain-specific, rat α1-tubulin (Tα1) is associated with the growth or remodeling of developing and mature neurons (Miller and Geddes, 1990). Whereas most highly expressed during embryonic development (Miller et al., 1987), Tα1-tubulin is expressed postnatally primarily in those regions of the brain most capable of undergoing growth and sprouting, including hippocampus and amygdala (Paden et al., 1995). In the adult brain, Tα1-tubulin is induced by a variety of stimuli (e.g., axotomy or seizure) that produce neuronal sprouting (Miller et al., 1987; Geddes et al., 1990; Represa et al., 1993). A correlation between Tα1-tubulin expression and neurite extension has also been observed in PC12 cells and in cultured sensory neurons treated with nerve growth factor (Miller et al., 1987; Mohiuddin et al., 1995).
The β-thymosin family of actin-binding proteins has also been shown to be involved in neuronal outgrowth (Safer and Nachmias, 1994). β-Thymosin has been shown to correlate with axonal growth in zebrafish CNS (Roth et al., 1999) and in Drosophila (Boquet et al., 2000), and seizures increase expression of β4 and β10 thymosin mRNA in rat brain (Carpintero et al., 1999). In Hermissenda, a β-thymosin homolog has been shown to be associated with an intermediate form of memory that requires translation but not transcription (Crow and Xue-Bian, 2000).
Our studies indicate that 5-HT stimulates translation of α-tubulin, an endogenous mRNA localized to the neurites, which has been shown to be translationally regulated in other species and cell types (Gonzalez-Garay and Cabral, 1996; Zheng et al., 1998). Previous studies have indicated that exogenously delivered mRNAs can be translated in neurites of individual neurons (Van Minnen et al., 1997; Spencer et al., 2000; Aakalu et al., 2001; Job and Eberwine, 2001). In dissected hippocampal slices, tetanic stimulation increases the dendritic translation of Ca2+/calmodulin kinase IIα (Ouyang et al., 1999) (but see Steward and Halpain, 1999). To our knowledge, this is the first demonstration of an endogenous mRNA being translationally regulated in a single neuron in response to synaptic stimulation.
The peripheral library has a large category of cDNAs involved in translation
More than 1/20 of the mRNAs present in our library encode molecules involved in translation (Table 1). Translation of most of these mRNAs is known to be rapamycin sensitive (Meyuhas and Hornstein, 2000), and we showed previously that an unusually large fraction of 5-HT-induced translation in neurites is rapamycin sensitive (Casadio et al., 1999). In fact, ribosomal protein mRNAs have been detected previously in synaptic compartments. Thus, in an independent study, Sossin and colleagues have shown that 5-HT increases S6 concentration in Aplysia synaptosomes (Khan et al., 2001), indicating that S6 mRNA is present at the synapse and that its translation is regulated by 5-HT. Kaplan and colleagues have also identified mRNAs encoding ribosomal proteins and translation factors in the giant axon of the squid (Gioio et al., 2001).
The synaptic translation of ribosomal proteins is especially surprising because ribosomal subunits are thought to be assembled in the nucleus (Leary and Huang, 2001). One possibility is that locally translated ribosomal proteins might perform extraribosomal functions (Wool, 1996). In analyzing the localization of these ribosomal proteins within the ribosome (Ban et al., 2000; Wimberly et al., 2000; Schlunzen et al., 2001; Spahn et al., 2001; Yusupov et al., 2001), we found that the proteins encoded by peripheral messages are generally located at the surface of the ribosome. This suggested to us that these locally synthesized ribosomal proteins could be added onto partially assembled ribosomes or could exchange with proteins on preexisting ribosomes. Ribosomal proteins stabilize and control the conformation of rRNA (Green and Noller, 1997), there is evidence for exchange of ribosomal proteins (Lastick and McConkey, 1976; Kruiswijk et al., 1978), and ribosomes can function in the absence of certain ribosomal proteins, although at lower efficiency (Dabbs, 1991). Furthermore, although the two ribosomal subunits are thought to be assembled in the nucleolus, the fully functioning 80 S ribosome is assembled in the cytoplasm when the large and small subunits come together in the process of translating an mRNA, and this process is mediated in part by ribosomal proteins (Spahn et al., 2001). Interestingly, our ribosomal protein mRNAs include L8 and S15, which provide such contacts between ribosomal subunits. Consistent with an important role in subunit bridging, one of these proteins, L8 (L2 in bacteria), is not required for the assembly of the large subunit itself but is required for the association of the small and large subunits in vitro (Diedrich et al., 2000). Thus, synthesis of these two ribosomal proteins could promote the assembly of a translationally competent 80 S ribosome at the synapse.
These data suggest that, in the absence of the ribosomal proteins encoded by local mRNAs in Aplysia neurites, ribosomal subunits would be translationally dormant, perhaps stored in RNA granules, large assemblies of translationally inactive ribosomes (Knowles et al., 1996; Krichevsky and Kosik, 2001). Such incomplete subunits would be unable to form functionally active ribosomes unless and until these proteins are added locally. We hypothesize that a few competent ribosomes at a synapse could make the missing ribosomal proteins, which could then allow incompetent subunits to be recruited from RNA granules to be assembled locally into translationally competent 80 S ribosomes. The presence in the peripheral library of two ubiquitin-ribosomal protein hybrid transcripts (ubiquitin-S27 and ubiquitin-L40) is also consistent with the possibility that synthesis of ribosomal proteins results in the local assembly of ribosomes, because the ubiquitin moieties have been found to facilitate assembly of these ribosomal proteins into ribosomes (Finley et al., 1989). The result would be an increase in the number of translationally competent ribosomes at those synapses at which synaptic stimulation had induced local protein synthesis. This complementation of partially assembled ribosomal subunits could be a mechanism for the local tagging of a synapse (Martin and Kosik, 2002), such that the generation of a localized site for translation would ensure that the products of transcription are only incorporated at stimulated synapses.
Locally expressed endogenous mRNA can be regulated by synaptic stimulation
Local protein synthesis is likely to prove to be a general mechanism used by neurons to spatially restrict gene expression. Thus, although our studies indicate that local translation plays an important role in learning-related synaptic plasticity, other studies have indicated that local translation underlies growth cone navigation during development (Campbell and Holt, 2001; Zhang and Poo, 2002) and axonal regeneration after injury (Zheng et al., 2001). In mature, fully polarized vertebrate neurons, translation is believed to occur exclusively postsynaptically, in the dendrite (Steward and Schuman, 2001). However, in immature, developing vertebrate neurons, there is clear evidence for local protein synthesis in both axonal and dendritic growth cones (Crino and Eberwine, 1996; Bassell et al., 1998; Brittis et al., 2002). Like other invertebrate neurons, Aplysia sensory cells do not develop fully distinct dendrites and axons but rather develop neurites, which have both receptive and transmissive properties (Mohr and Richter, 1993). We thus believe that the cytoskeletal and translational mRNAs present in our library will be important to many processes in which local protein synthesis contributes to synapse formation and strengthening in neurons.
Footnotes
This work was supported by the Howard Hughes Medical Institute (E.R.K.), National Institutes of Health (NIH) (K.C.M.), an NIH MSTP grant (R.M.), the Burroughs Wellcome Fund (K.C.M.), the McKnight Foundation (K.C.M.), the Klingenstein Fund (K.C.M.), and the W. M. Keck Foundation (K.C.M.). We thank Huixiang Zhu and Defang Ma for Aplysia neuronal cultures, Natalie Digate for assistance in library screening, Catalina Wang for assistance in image acquisition and analysis, Kausik Si for comments on a previous version of this manuscript, and members of the Martin-Barad laboratory for helpful discussions.
Correspondence should be addressed to Kelsey C. Martin, Gonda 3506/BRI, University of California, Los Angeles, 695 Charles Young Drive South, Los Angeles, CA 90095-1761. E-mail: kcmartin@mednet.ucla.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/239409-09$15.00/0
R.M. and D.C. contributed equally to this work.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM ( 2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30: 489-502. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER ( 1994) C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia Cell 76: 1099-1114. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ ( 1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M ( 1983) Morphological basis of long-term habituation and sensitization in Aplysia Science 220: 91-93. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M ( 1988) Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci USA 85: 9356-9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA ( 2000) The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289: 905-920. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER ( 1998) CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95: 211-223. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS ( 1998) Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci 18: 251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet I, Boujemaa R, Carlier MF, Preat T ( 2000) Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell 102: 797-808. [DOI] [PubMed] [Google Scholar]
- Brady G, Iscove NN ( 1993) Construction of cDNA libraries from single cells. Methods Enzymol 225: 611-623. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG ( 2002) Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110: 223-235. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Shapiro E, Foster SA, Kandel ER, Iino Y ( 1991) Identification of a peptide specific for Aplysia sensory neurons by PCR-based differential screening. Science 252: 856-859. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE ( 2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32: 1013-1026. [DOI] [PubMed] [Google Scholar]
- Carpintero P, Anadon R, Diaz-Regueira S, Gomez-Marquez J ( 1999) Expression of thymosin beta4 messenger RNA in normal and kainate-treated rat forebrain. Neuroscience 90: 1433-1444. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER ( 1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221-237. [DOI] [PubMed] [Google Scholar]
- Crino PB, Eberwine J ( 1996) Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron 17: 1173-1187. [DOI] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ ( 2000) Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda J Neurosci 20: RC74(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs ER ( 1991) Mutants lacking individual ribosomal proteins as a tool to investigate ribosomal properties. Biochimie 73: 639-645. [DOI] [PubMed] [Google Scholar]
- Diedrich G, Spahn CM, Stelzl U, Schafer MA, Wooten T, Bochkariov DE, Cooperman BS, Traut RR, Nierhaus KH ( 2000) Ribosomal protein L2 is involved in the association of the ribosomal subunits, tRNA binding to A and P sites and peptidyl transfer. EMBO J 19: 5241-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Axel R ( 1995) A novel family of genes encoding putative pheromone receptors in mammals. Cell 83: 195-206. [DOI] [PubMed] [Google Scholar]
- Eberwine J, Belt B, Kacharmina JE, Miyashiro K ( 2002) Analysis of subcellularly localized mRNAs using in situ hybridization, mRNA amplification, and expression profiling. Neurochem Res 27: 1065-1077. [DOI] [PubMed] [Google Scholar]
- Finley D, Bartel B, Varshavsky A ( 1989) The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature 338: 394-401. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Wong J, Choi BH, Kim RC, Cotman CW, Miller FD ( 1990) Increased expression of the embryonic form of a developmentally regulated mRNA in Alzheimer's disease. Neurosci Lett 109: 54-61. [DOI] [PubMed] [Google Scholar]
- Gioio AE, Eyman M, Zhang H, Lavina ZS, Giuditta A, Kaplan BB ( 2001) Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J Neurosci Res 64: 447-453. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S ( 1990) Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science 249: 799-802. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garay ML, Cabral F ( 1996) alpha-Tubulin limits its own synthesis: evidence for a mechanism involving translational repression. J Cell Biol 135: 1525-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Noller HF ( 1997) Ribosomes and translation. Annu Rev Biochem 66: 679-716. [DOI] [PubMed] [Google Scholar]
- Hall JL, Cowan NJ ( 1985) Structural features and restricted expression of a human alpha-tubulin gene. Nucleic Acids Res 13: 207-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C, Eberwine J ( 2001) Identification of sites for exponential translation in living dendrites. Proc Natl Acad Sci USA 98: 13037-13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Pepio AM, Sossin WS ( 2001) Serotonin activates S6 kinase in a rapamycin-sensitive manner in Aplysia synaptosomes. J Neurosci 21: 382-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GJ, Kosik KS ( 1996) Translocation of RNA granules in living neurons. J Neurosci 16: 7812-7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS ( 2001) Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32: 683-696. [DOI] [PubMed] [Google Scholar]
- Kruiswijk T, Planta RJ, Krop JM ( 1978) The course of the assembly of ribosomal subunits in yeast. Biochim Biophys Acta 517: 378-389. [DOI] [PubMed] [Google Scholar]
- Lastick SM, McConkey EH ( 1976) Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem 251: 2867-2875. [PubMed] [Google Scholar]
- Leary DJ, Huang S ( 2001) Regulation of ribosome biogenesis within the nucleolus. FEBS Lett 509: 145-150. [DOI] [PubMed] [Google Scholar]
- Lockhart ST, Levitan IB, Pikielny CW ( 1996) Ag, a novel protein secreted from Aplysia glia. J Neurobiol 29: 35-48. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kandel ER ( 1996) Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron 17: 567-570. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kosik KS ( 2002) Synaptic tagging—who's it? Nat Rev Neurosci 3: 813-820. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER ( 1997a) MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia Neuron 18: 899-912. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, E Y, Rose JC, Chen M, Bailey CH, Kandel ER ( 1997b) Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91: 927-938. [DOI] [PubMed] [Google Scholar]
- Meyuhas O, Hornstein E ( 2000) Translational control of TOP mRNAs. In: Translational control of gene expression (Sonenberg N, Hershey JWB, Mathews MB, eds), pp 671-694. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER ( 1998) Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia Proc Natl Acad Sci USA 95: 1864-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FD, Geddes JW ( 1990) Increased expression of the major embryonic alpha-tubulin mRNA, T alpha 1, during neuronal regeneration, sprouting, and in Alzheimer's disease. Prog Brain Res 86: 321-330. [DOI] [PubMed] [Google Scholar]
- Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ ( 1987) Isotypes of alpha-tubulin are differentially regulated during neuronal maturation. J Cell Biol 105: 3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin L, Fernandez K, Tomlinson DR, Fernyhough P ( 1995) Nerve growth factor and neurotrophin-3 enhance neurite outgrowth and up-regulate the levels of messenger RNA for growth-associated protein GAP-43 and T alpha 1 alpha-tubulin in cultured adult rat sensory neurones. Neurosci Lett 185: 20-23. [DOI] [PubMed] [Google Scholar]
- Mohr E, Richter D ( 1993) Dendritic and axonal mRNA trafficking. Regul Pept 45: 21-24. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S ( 1986) A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia Science 234: 1249-1254. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB ( 1999) Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J Neurosci 19: 7823-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden CM, Zhou X, Watt JA, Burton R, Pickett J, Oblinger MM ( 1995) Distribution of growth-associated class I alpha-tubulin and class II beta-tubulin mRNAs in adult rat brain. J Comp Neurol 362: 368-384. [DOI] [PubMed] [Google Scholar]
- Represa A, Pollard H, Moreau J, Ghilini G, Khrestchatisky M, Ben-Ari Y ( 1993) Mossy fiber sprouting in epileptic rats is associated with a transient increased expression of alpha-tubulin. Neurosci Lett 156: 149-152. [DOI] [PubMed] [Google Scholar]
- Roth LW, Bormann P, Bonnet A, Reinhard E ( 1999) beta-thymosin is required for axonal tract formation in developing zebrafish brain. Development 126: 1365-1374. [DOI] [PubMed] [Google Scholar]
- Safer D, Nachmias VT ( 1994) Beta thymosins as actin binding peptides. BioEssays 16: 590. [DOI] [PubMed] [Google Scholar]
- Schlunzen F, Zarivach R, Harms J, Bashan A, Tocilj A, Albrecht R, Yonath A, Franceschi F ( 2001) Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 413: 814-821. [DOI] [PubMed] [Google Scholar]
- Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J ( 2001) Structure of the 80S ribosome from Saccharomyces cerevisiae- tRNA-ribosome and subunit-subunit interactions. Cell 107: 373-386. [DOI] [PubMed] [Google Scholar]
- Spencer GE, Syed NI, van Kesteren E, Lukowiak K, Geraerts WP, van Minnen J ( 2000) Synthesis and functional integration of a neurotransmitter receptor in isolated invertebrate axons. J Neurobiol 44: 72-81. [DOI] [PubMed] [Google Scholar]
- Steward O, Halpain S ( 1999) Lamina-specific synaptic activation causes domain-specific alterations in dendritic immunostaining for MAP2 and CAM kinase II. J Neurosci 19: 7834-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM ( 2001) Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci 24: 299-325. [DOI] [PubMed] [Google Scholar]
- Van Minnen J, Bergman JJ, Van Kesteren ER, Smit AB, Geraerts WP, Lukowiak K, Hasan SU, Syed NI ( 1997) De novo protein synthesis in isolated axons of identified neurons. Neuroscience 80: 1-7. [DOI] [PubMed] [Google Scholar]
- Wimberly BT, Brodersen DE, Clemons Jr WM, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V ( 2000) Structure of the 30S ribosomal subunit. Nature 407: 327-339. [DOI] [PubMed] [Google Scholar]
- Wool IG ( 1996) Extraribosomal functions of ribosomal proteins. Trends Biochem Sci 21: 164-165. [PubMed] [Google Scholar]
- Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF ( 2001) Crystal structure of the ribosome at 5.5 A resolution. Science 292: 883-896. [DOI] [PubMed] [Google Scholar]
- Zhang X, Poo MM ( 2002) Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 36: 675-688. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL ( 2001) A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci 21: 9291-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Roy PJ, Liang P, MacRae TH ( 1998) Cloning and sequencing of an alpha-tubulin cDNA from Artemia franciscana: evidence for translational regulation of alpha-tubulin synthesis. Biochim Biophys Acta 1442: 419-426. [DOI] [PubMed] [Google Scholar]