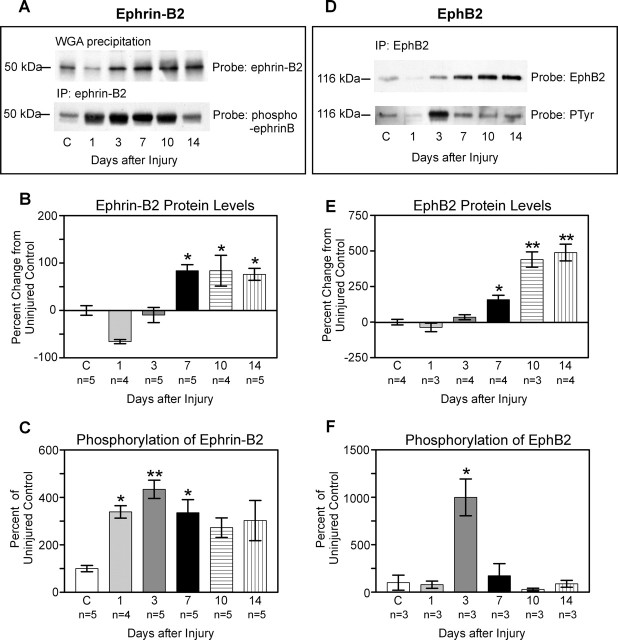
Figure 3.
Western blots and quantification of protein and phosphorylation levels at progressive time points (1, 3, 7, 10, and 14 d) after complete T7 transections of the spinal cord. A, Representative Western blot illustrating temporal changes in ephrin-B2 protein and phosphorylation. Ephrin-B2 proteins were precipitated with WGA, and blots were probed with anti-ephrin-B2 (top). Ephrin-B2 protein was immunoprecipitated (IP) with anti-ephrin-B2, and resulting Western blots were probed with an antibody recognizing phosphorylated B-ephrins (bottom). B, Quantification of ephrin-B2 protein. At 1 d after lesion, there was a decrease in ephrin-B2 of ∼66% from control (C) levels. Ephrin-B2 protein quickly rebounded to near control levels by 3 d and exhibited a significant increase of 76-84% above uninjured levels from 7 to 14 d after lesion. Protein levels 7, 10, and 14 d after lesion were not significantly different from one another but were significantly different (*) from uninjured control (p < 0.05), from 1 d after lesion (p < 0.001), and 3 d after lesion (p < 0.01; Tukey's post hoc test). C, Quantification of ephrin-B2 phosphorylation. Low levels of ephrin-B2 phosphorylation were detected in uninjured tissue. At 1 d after lesion, there was a rapid and significant activation of ephrin-B2 compared with control (339%; p < 0.05), which additionally increased 3 d after lesion (435%; p < 0.01). Phosphorylation levels remained elevated at 7 d (335%; p < 0.05) before slowly decreasing. D, Representative Western blot illustrating temporal changes in EphB2 protein and phosphorylation. EphB2 protein was immunoprecipitated with anti-EphB2, and resulting Western blots were probed with anti-EphB2 (top). Activated EphB2 receptors were identified by immunoprecipitating EphB2 and probing the resulting Western blots with anti-phosphotyrosine (PTyr; bottom). E, Quantification of EphB2 protein. As for ephrin-B2, there was an initial drop in EphB2 protein at 1 d, which was followed by an increase that reached ∼160% of uninjured levels by day 7. At both 10 and 14 d, there was a highly significant increase in EphB2 protein of >400% from uninjured levels. Protein levels 10 and 14 d after lesion were significantly different (**) from uninjured, 1, 3 (p < 0.001), and 7 (p < 0.01) d. Protein levels 7 d after lesion were significantly different (*) from levels 1 d after lesion (p < 0.05). F, Quantification of EphB2 phosphorylation. A low level of EphB2 phosphorylation was detected in uninjured spinal cord tissue. At 3 d after injury, there was a highly significant and transient increase (1000%; p < 0.001) in EphB2 phosphorylation.