Figure 8.
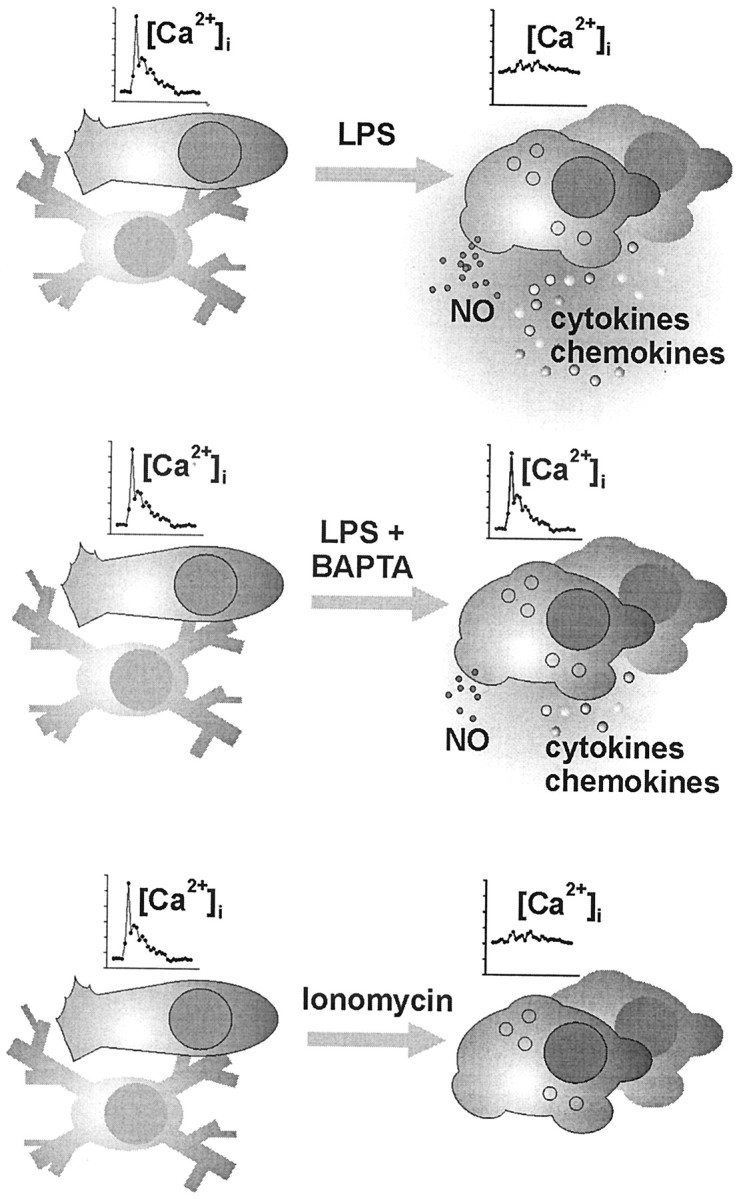
Schematic summary of the experimentally induced adaptations of microglia. Unstimulated microglial cells (shown on the left) in vitro typically appearing with a short-processed, nonbranched or rod-shaped morphology responded to UTP and C5a receptor stimulation with the generation of a [Ca 2+]i transient but did not show signs of release activity, similar to the “resting” ramified microglia of the normal brain tissue. A challenge of these cells under pathological conditions, as mimicked here by a treatment with bacterial LPS, led to a complex morphological and functional transformation known as microglial activation. In the present study, LPS-activated cells were characterized by an elevated basal [Ca 2+]i level along with a suppressed ability to generate receptor-triggered [Ca 2+]i signals. Strong release of NO as well as cytokines and chemokines was measured. LPS treatment in the presence of BAPTA nearly restored the receptor signaling efficacy, paralleled by an almost back-to-normal basal [Ca 2+]iand an attenuated release performance. On the otherhand, direct enforcement of a rise in basal [Ca 2+]i by microglial incubation with ionomycin also suppressed the signal amplitudes but failed to trigger an LPS-like release pattern. Together, these observations suggest that an elevated basal [Ca 2+]i participates in the functional adjustment of activated microglia, without being the sole cytosolic control element for executive features, such as NO and cytokine–chemokine production.
