Figure 8.
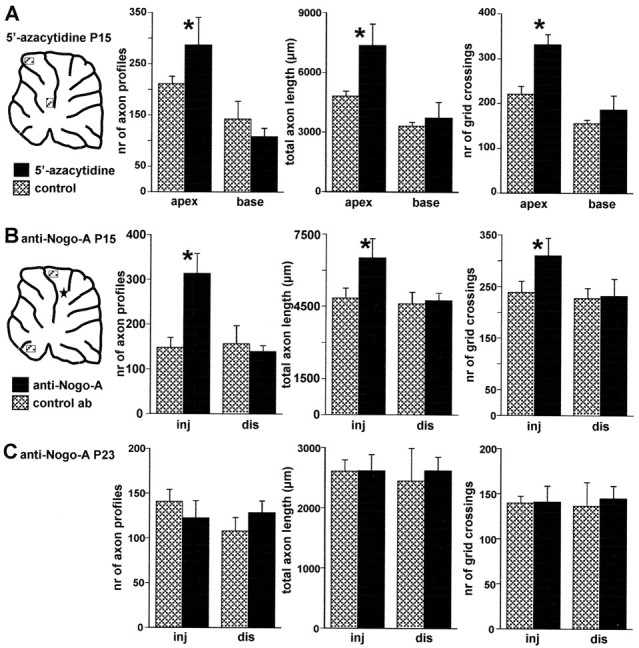
Morphometric evaluation of Purkinje axon intracortical plexus in 5′-azacytidine- and anti-Nogo-A-treated animals. A, Different morphometric parameters evaluated in selected areas from basal and apical regions of cerebellar lobules from 5′-azacytidine-treated animals (solid bars, mean ± SD; n = 5) and vehicle-injected control animals (hatched bars, mean ± SD; n = 5; the diagram shows the approximate position of the examined areas). All parameters are significantly increased in the apical regions, whereas no differences occur when basal regions are compared. B, Similar analysis performed on anti-Nogo-A-treated rats (solid bars, mean ±SD; n = 5) or control antibody-treated rats (hatched bars, mean ±SD; n = 4) killed at P15 (5 d after antibody injection). In this case, comparisons were made between areas from lobules near the injection site (inj) and areas from distant lobules (dis) in the ventral cerebellum. The diagram shows the approximate position of the examined areas and of the injection site, indicated by the star. A significant increase of all morphometric parameters occurs in the vicinity of anti-Nogo-A application site, whereas no differences occur in the distant regions. C, Results of the same analysis performed on animals killed at P23 (13 d after antibody injections). There are no significant differences between anti-Nogo-A (solid bars, mean ± SD; n = 4) and control antibody treated animals (hatched bars, mean ± SD; n = 4), indicating that the effect of anti-Nogo-A antibodies are transitory. Asterisks indicate values that are significantly different from the relevant control (see Results).
