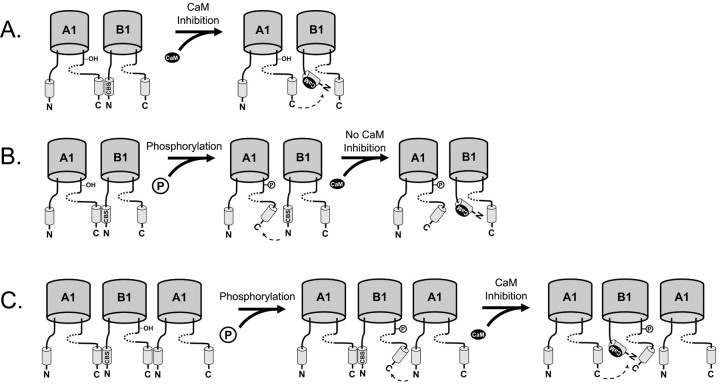
Figure 6.
Proposed mechanism for suppression of Ca2+/CaM inhibition of rod CNG channels by phosphorylation of CNGA1Y498. A, Ca2+/CaM inhibits rod CNG channels by binding to a Ca2+/CaM binding-site (CBS) on CNGB1 (B1) and disrupting its interaction with the C terminus of CNGA1 (A1) (Trudeau and Zagotta, 2002). B, Phosphorylation of CNGA1Y498 also disrupts the interaction between the C terminus (C) of CNGA1 and the N terminus (N) of CNGB1. Thus, when Ca2+/CaM binds the CBS, there is no additional effect on the intersubunit interaction. C, Phosphorylation of CNGB1Y1097 may disrupt a similar interface between the C-terminus region of CNGB1 and the N-terminus region of CNGA1. Binding of Ca2+/CaM to the Ca2+/CaM binding-site can still disrupt the interface between the C terminus of CNGA1 and the N terminus of CNGB1. Hence, phosphorylation of CNGB1Y1097 has no effect on Ca2+/CaM inhibition. The cyclic-nucleotide binding domain is shown as a dotted line.