Abstract
The molecular mechanisms mediating degeneration of midbrain dopamine neurons in Parkinson's disease (PD) are poorly understood. Here, we provide evidence to support a role for the involvement of the calcium-dependent proteases, calpains, in the loss of dopamine neurons in a mouse model of PD. We show that administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) evokes an increase in calpain-mediated proteolysis in nigral dopamine neurons in vivo. Inhibition of calpain proteolysis using either a calpain inhibitor (MDL-28170) or adenovirus-mediated overexpression of the endogenous calpain inhibitor protein, calpastatin, significantly attenuated MPTP-induced loss of nigral dopamine neurons. Commensurate with this neuroprotection, MPTP-induced locomotor deficits were abolished, and markers of striatal postsynaptic activity were normalized in calpain inhibitor-treated mice. However, behavioral improvements in MPTP-treated, calpain inhibited mice did not correlate with restored levels of striatal dopamine. These results suggest that protection against nigral neuron degeneration in PD may be sufficient to facilitate normalized locomotor activity without necessitating striatal reinnervation. Immunohistochemical analyses of postmortem midbrain tissues from human PD cases also displayed evidence of increased calpain-related proteolytic activity that was not evident in age-matched control subjects. Taken together, our findings provide a potentially novel correlation between calpain proteolytic activity in an MPTP model of PD and the etiology of neuronal loss in PD in humans.
Keywords: substantia nigra, dopamine, neurotensin, FosB, protease, adenovirus, behavior, l-Dopa
Introduction
The molecular events responsible for the loss of dopaminergic neuron in the substantia nigra pars compacta (SNc) in Parkinson's disease (PD) remain poorly understood. One prominent feature of PD is a deficiency in mitochondrial function attributed to reduced complex 1 activity in the SNc (Schapira et al., 1989; Greenamyre et al., 2001). Experimentally, administration of chemical inhibitors of complex 1 of the mitochondrial respiration chain can mimic key features of PD, including the selective dopaminergic neuropathology and behavioral deficits (Beal, 2001). These findings support accumulating evidence that nigral dopamine neurons are highly sensitive to stress related to reduced mitochondrial function.
The cellular consequences of deficits in mitochondrial function include reduced ATP production (Greenamyre et al., 1999), oxidation-related changes in protein function (Jenner, 1998; Przedborski et al., 2003), and poor calcium homeostasis (Sheehan et al., 1997; Sherer et al., 2001). It is of particular relevance to this latter point that previous work has also shown that N-methyl-4-pyridinium (MPP+), the active metabolite of the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), can evoke a sustained elevation of cytoplasmic calcium levels (Chen et al., 1995). This change in calcium likely occurs through several processes, including secondary excitotoxic mechanisms and depletion of mitochondrial calcium pools (Frei and Richter, 1986; Kass et al., 1988). Such abnormal calcium homeostasis manifests activation of various intracellular signaling pathways that impact on neuronal function and survival (Wang and Yuen, 1994). Although the effects of increased intracellular calcium in nigral dopamine neurons is almost certainly complex, one consequence may be the activation of the calcium-sensitive proteases, calpains.
Calpains are a highly conserved family of calcium-dependent proteases. There are two ubiquitously expressed calpain isoforms, μ (calpain-1) and m (calpain-2). Each calpain is composed of a unique large subunit and a common small regulatory subunit (for review, see Sorimachi et al., 1997). The importance of calpains is underscored by the observation that mice deficient in the 30 kDa small regulatory subunit suffer embryonic lethality (Arthur et al., 2000; Zimmerman et al., 2000). In the CNS, calpains are widely expressed (Goto et al., 1994; Li et al., 1996) and modulated by an endogenously expressed inhibitory protein, calpastatin (Emori et al., 1987; Wang and Yuen, 1994).
The role of calpains in PD is unknown. In human PD tissues, it has been reported that expression of m-calpain is increased in dopamine neurons (Mouatt-Prigent et al., 1996); however, the significance of this observation is not clear. Here, we provide evidence to support a role for calpain activation as a process mediating the loss of nigral dopamine neurons in a mouse model of PD and demonstrate that inhibition of calpains prevents reduced motor function in mice through normalization of basal ganglia (BG) activity, albeit in the absence of restored striatal dopamine. We also provide evidence that calpain activity is enhanced in nigral dopamine neurons of postmortem tissues from human PD cases. These findings support a novel role for calpains in the molecular events related to nigral neuron dysfunction in PD.
Materials and Methods
Mice
All procedures involving animals were approved by the University of Ottawa Animal Care Committee and maintained in strict accordance with the Guidelines for the Use and Treatment of Animals put forth by the Animal Care Council of Canada and endorsed by the Canadian Institutes of Health Research.
MPTP
N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (25 mg/kg, i.p., measured as free base; MPTP-HCl; Sigma, St. Louis, MO) was administered to male C57BL/6 mice (8-10 weeks old; Charles River Laboratories, St. Constant, Quebec) once a day for 5 consecutive days (Tatton and Kish, 1997; Xia et al., 2001) (Fig. 1 A). Mice used as controls received an equivalent volume of saline (0.9%) once daily.
Figure 1.
Schematic representation of time course and experimental manipulations for each set of experiments in this study. Each horizontal line represents the duration of each experiment. Numerals above the horizontal line indicate time point (in days) of experimental end-points, whereas the set of five vertical lines indicates the timing of MPTP dosing (25 mg/kg measured as free base, i.p., per day for 5 consecutive days). A, Mice used for experiments examining the time course of calpain activation after MPTP intoxication were taken either before MPTP (day 0) or 7 or 14 d after the first injection (+7, +14). B, Mice implanted with osmotic minipumps (Alza) 1 d (-1) before the initiation of the MPTP regime (0). In this set of experiments, the locomotor behavior of these mice was analyzed 1 d before (+13) the 2 week end point (+14), and additional groups were also analyzed for survival at 3 weeks (+21). C, Recombinant adenoviruses were administered 1 week (-7) before the start of MPTP dosing (0), and tissues were analyzed 2 weeks later (+14). See Materials and Methods for details.
Calpain inhibition
Osmotic minipumps (Alza, Palo Alto, CA; model 1007D) were implanted into the right lateral ventricle 1.0 mm rostral and 2.2 mm to the right of bregma with the cannula extending to a depth of 2.5 mm from the skull surface. Pumps were prefilled with 200 μl of either vehicle (Krebs'-Ringer's solution) or MDL-28170 (160 μm; carbobenzylzoxy-Val-Phe-H) and implanted 24 hr before the start of the MPTP dosing regimen (Fig. 1 B). MDL-28170 was diluted in vehicle containing 10% cycylodextrins (RBI, Natick, MA) from a 20 mm stock solution (in dimethyl sulfoxide). Behavioral analyses and assessment of nigral dopamine neuron survival in all osmotic pump-implanted mice was performed 2 or 3 weeks after the initiation of MPTP.
MPP+ measurement
Twenty-four hours after the implantation of osmotic pumps delivering either MDL-28170 or vehicle (see above), striatal concentrations of MPP+ were measured 90 min after a single injection of MPTP. HPLC measurements were performed as described previously (Przedborski et al., 1996).
Adenoviral gene delivery
Additional groups of mice were administered recombinant adenoviruses that expressed either the calpain inhibitor protein calpastatin or the bacterial reporter gene lacZ. Calpastatin (RNCAST104) (Melloni et al., 1998) was excised from pGEX 6P1 using BamH1 and EcoR1 and ligated into the pAd-lox vector for amplification into recombinant adenovirus, as described (Hardy et al., 1997). Adenoviruses (3 μl; 1 × 10 7 particles μl -1 per construct) expressing either calpastatin (Ad.CALP) or a control expression marker, lacZ (Ad.lacZ), were stereotaxically injected into the right dorsolateral striatum (0.5 mm rostral and 2.2 mm to the right of bregma and 3.4 mm below the skull surface) at an infusion rate of 0.5 μl/min using a syringe pump (PHD2000, Harvard Apparatus, St. Laurent, Quebec). Mice were challenged with MPTP 1 week after adenovirus injection (Fig. 1C) to permit sufficient time for retrograde transport and expression of the adenovirus-derived proteins. Assessment of dopamine neuron survival was made 2 weeks after the start of the MPTP dosing regimen.
Immunohistochemistry
Brain tissues from mice injected with MPTP or saline were collected for immunohistochemical analyses as described previously (Crocker et al., 2001a,2001b). Antibodies used were tyrosine hydroxylase (TH) (1:1000; Inc-Star), dopamine transporter (DAT) (1:5000; Chemicon), and calpastatin [1:500 (Melloni et al., 1998)]. Calpain activity was detected using an antibody generated against a peptide fragment of α-spectrin derived by calpain-mediated cleavage (38-4, 1:2000), which is highly selective and specific for calpain-cleaved fragments of α-spectrin but not for α-spectrin cleavage by other proteases (Roberts-Lewis et al., 1994). Immunoreactivity was visualized using an avidin-biotin complex peroxidase reaction (Crocker et al., 1998). Immunofluorescent labeling was visualized using secondary antibodies (Jackson Labs): anti-mouse IgG-conjugated CY3 antibody for detection of TH (Jackson Labs) and anti-rabbit IgG or anti-goat IgG conjugated to FITC for detection of 38-4 and calpastatin, respectively.
Assessment of neuronal loss
Loss of neurons in the SNc was determined by serial section analysis of the total number of TH-positive (TH+) neurons. Every sixth coronal section throughout the entire rostral-caudal (RC) axis of the murine SNc was collected for assessment of neuronal survival by immunohistochemistry (Franklin and Paxinos, 1997). Adjacent SNc tissue sections from each animal were also stained with cresyl violet to validate immunohistochemical determination of nigral neuron survival. Estimates of total TH+ and cresyl-stained nigral neuron populations were calculated using Abercrombie's correction (Abercrombie, 1946).
For those experiments in which adenoviruses were used, only the sections within the range of the medial terminal nucleus (MTN) were evaluated, because intrastriatal administration results in retrograde labeling of only a subpopulation of SNc neurons (Crocker et al., 2001a). The total numbers of TH+ neurons (-3.08 to -3.28 mm) in the ipsilateral and contralateral hemispheres were counted separately from at least six sections for each animal. Treatment groups were averaged and differences were analyzed by a one-way ANOVA, followed by Newman-Keuls test, and considered significant when p < 0.05.
Striatal densitometry
Quantification of striatal dopaminergic fibrous staining and striatal FosB-positive nuclei was performed on striatal tissues from animals 14 d after the start of MPTP. Counts were made by sampling an area 660 × 800 μm in at least five sections per animal using computer-assisted image analysis software (Northern Eclipse, Empix Imaging, Mississauga, ON), as described previously (Crocker et al., 2001a,2001b). Analyses were performed by an individual unaware of the experimental treatments.
Striatal HPLC
Levels of neurotransmitters and metabolites were separated and measured simultaneously from single perchloric acid (PCA) extracts using HPLC with electrochemical detection as described previously (Hayley et al., 1999; Crocker et al., 2001a). Extracts were taken from groups of mice 2 weeks after the start of MPTP injections.
Behavioral analyses
Behavioral analyses were performed to assess locomotor function 14 d after the start of MPTP dosing (Fig. 1 B).
Novel environment. Walled 32-× 32-inch-square arenas were used for open field testing of mice, using a video camera and analysis software (Videomex 5, Columbus Instruments). Total horizontal ambulatory distance traveled during the 1 hr observation period was reported in centimeters. Additional groups of unlesioned and MPTP-lesioned (VEH-MPTP) mice were also administered l-Dopa (15 mg/kg, i.p.) to determine whether the locomotor deficit in this novel environment was reversible with dopamine replacement.
Home cage. Additional groups of animals were analyzed for behavioral performance in response to amphetamine administration (2.0 mg/kg, s.c.) using activity cages outfitted with infrared detector arrays, as described previously (Merali and Piggins, 1990). Home cages were used for amphetamine (2.0 mg/kg)-induced activity to exclude the influence of a novel environment on behavioral performance. All assessments of behavioral performance were performed by individuals blinded to the experimental treatments.
Striatal neurotensin radioimmunoassay
Using a modification of the method of Palkovits (Palkovits and Brownstein, 1988), serial coronal cryostat sections were used to micropunch (Micro Punch MP-600, ASI Instruments) striatal samples from tissues from each subject of each treatment group. Detection and quantification of neurotensin (NT) were achieved through a high-sensitivity double-antibody liquid-phase RIA kit obtained through Phoenix Pharmaceuticals (Belmont, CA). A four-parameter logistic curve fit model was used for interpolation of the standard curves. Sensitivity of the assay had an IC50 = 19.1 pg per tube. Tissues were extracted from mice 14 d after the start of MPTP (n = 7-8 per group).
Human brain samples
Paraffin-embedded postmortem human tissues from Parkinson's disease brains and control brains were provided by the Harvard Brain Tissue Resource Center (HBTRC) and were for analysis of calpain-related gene expression (Table 1). The age and postmortem interval from matched samples did not differ (p < 0.25). Diagnoses were made on the basis of medical histories and postmortem confirmation by HBTRC. Of the PD subjects, the average age of symptom onset was 52.8 ± 5.8 years, whereas the average duration of illness was 15.6 ± 3.9 years. Pharmacotherapy for all cases included l-Dopa or Sinemet, and Eldepryl (Table 2).
Table 1.
Age and postmortem interval (PMI) of matched human nigral tissue samples
|
Sample number |
Disease condition |
Age |
PMI (hr) |
|---|---|---|---|
| 1 | Parkinson's disease | 63 | 2.7 |
| 2 | Parkinson's disease | 83 | 5.5 |
| 3 | Parkinson's disease | 73 | 8.2 |
| 4 | Parkinson's disease | 78 | 13.5 |
| Average 74.3 | Average 7.5 | ||
| 5 | Control | 85 | 4 |
| 6 | Control | 76 | 5.4 |
| 7 | Control | 68 | 14.8 |
| 8 | Control | 65 | 17.3 |
| 9 | Control | 70 | 18 |
|
|
|
Average 72.8
|
Average 11.9
|
Table 2.
Summary of clinical case histories of Parkinson's disease cases used in our study based on available records from Harvard Brain Bank
|
Case number |
Age of diagnosis |
Duration of illness |
Medications |
Cause of death |
|---|---|---|---|---|
| 1 | 59 | 4 years | a,b,c,d,e,f | Cardiopulmonary arrest |
| 2 | 68 | 15 years | a,h,n,p,q,r,s,t,u,v w,x,y,z,aa,bb,cc,dd ee,ff,gg | Congestive heart failure |
| 3 | 45 | 28 years | c,g,h,l,j,k,l,m | Dehydration |
| 4
|
66
|
12 years
|
a,n,o
|
Epidural hematoma
|
Medications: a, Sinemet; b, Propanolol; c, Parlodel; d, Eldepryl; e, Prozac; f, Diphenhydramine; g, l-Dopa; h, Zantac; i, Ditropan; j, Symmetrel; k, Xanax; l, Halclon; m, Deprenyl; n, Permax; o, Docusate calcium; p, Amitripyline; q, Ativan; r, Bactroban; s, Blephamide; t, Opthalmic cormax; u, Dicloxacillin; v, Digoxin; w, Fentany; x, Furosemide; y, Ibuprofen; z, Lanoxin; aa, Lasix; bb, morphine; cc, Olanzepine; dd, Prednisone; ee, Salicyclic acid; ff, Vasotec; gg, Zaroxlolyn.
Because the absolute number of melanized dopamine neurons varies between each individual, the number of immunopositive neurons was calculated as a proportion of the number of pigmented neurons for each section and subject (F = 55.95; p < 0.0001). Hence, determinations of significance between the estimated proportion of immunopositive dopamine neurons in the PD subjects were compared with the control cases using a one-tailed Mann-Whitney rank sum test (Hirsch et al., 1988; Hartmann et al., 2001). Differences were considered significant when p < 0.05.
Results
MPTP evokes sustained activation of calpains in the SNc
We first determined whether calpains may be activated in SNc neurons after chronic MPTP treatment in mice. Using an anti-body that selectively recognizes an epitope within α-spectrin that has been exposed by calpain proteolysis (neoepitope) (Roberts-Lewis et al., 1994), midbrain sections from mice treated with MPTP were analyzed for evidence of increased calpain-mediated proteolysis by immunohistochemistry. Mice that were treated with saline did not exhibit any detectable increase in neoepitope labeling (Fig. 2a), whereas a noticeable and sustained increase in calpain-mediated proteolysis was observed in the nigral region of mice that had been treated with MPTP (Fig. 2b,c). Closer examination of this region revealed that the pattern of calpain-cleaved α-spectrin was located predominantly in the cytoplasm and was punctate in appearance (Fig. 2e,f). This punctate appearance resembled calpain translocation to intracellular membrane compartments in hippocampal neurons after ischemia in vivo (Yamashima et al., 1998). Intracerebroventricular infusion of the pharmacological calpain inhibitor MDL-28170 prevented the emergence of immunoreactivity for calpain-cleaved α-spectrin after chronic MPTP administration (Fig. 2d).
Figure 2.
Increased calpain proteolysis in nigral dopamine neurons of MPTP-treated mice. Detection of calpain-cleaved α-spectrin was absent in the SNc of saline-treated mice (a). Administration of MPTP (25 mg/kg measured as free base, i.p.) daily for 5 consecutive days resulted in increased reactivity for calpain-cleaved α-spectrin 7 d (b) and 14 d (c) later. Intracerebroventricular coadministration of the calpain inhibitor MDL-28170 blocked MPTP-induced calpain-mediated cleavage of α-spectrin 14 d after MPTP (d). MPTP-treated mice that were coadministered the vehicle (e, f) also revealed a predominance of punctate and cytoplasmic patterns of immunostaining for calpain-cleaved α-spectrin, whereas nuclear immunostaining was weak and not pronounced (f, inset). Immunohistofluorescence confirmed the increased expression of calpain-cleaved α-spectrin (g, i) in tyrosine hydroxylase-immunoreactive cell bodies in the substantia nigra (h, j) of MPTP-treated mouse brain tissues 14 d (i, j) after treatment, but not in the SNc of saline-treated mice (g, h). Clusters of speckled immunoreactivity were nonreactive for glial acidic fibrillary protein (data not shown). Arrowheads indicate region of the SNc (a-d), and arrow in e indicates area magnified in cell inset (f). Scale bars: a-e, 100 μm; g-j, 15 μm; f, inset, 25 μm.
To demonstrate that the observed increase in calpain-related activity indeed was in nigral dopamine neurons, we performed immunofluorescent double-labeling experiments using TH as the dopaminergic marker. In midbrain tissues from untreated mouse brains, calpain-cleaved α-spectrin immunostaining was weak and diffusely cytoplasmic in nigral dopaminergic neurons (Fig. 2g,i). However, in tissues from mice treated with MPTP, this cytoplasmic immunolocalization within dopamine neurons exhibited a bright speckled appearance that became progressively more intense by 14 d (Fig. 2h,j) after cessation of MPTP administration.
Calpain inhibition prevents nigral dopamine neuron degeneration
To examine whether the increased calpain-mediated proteolytic activity was related to the loss of dopamine neurons after MPTP administration, we infused mice with either the calpain inhibitor (MDL-28170) or its vehicle (VEH) and then treated them with MPTP. This pharmacological calpain inhibitor has been previously reported to attenuate neuronal loss after transient ischemia (Markgraf et al., 1998). Exposure of mice to MPTP produced a substantial loss of nigral dopamine neurons (Fig. 3b) when compared with unlesioned controls (Fig. 3a, Table 3). However, intracerebroventricular administration of the calpain inhibitor MDL-28170 significantly attenuated the loss of nigral dopamine neurons 2 weeks after the initiation of MPTP treatment (Fig. 3c). Analysis of the distribution of nigral dopamine (TH+) neurons along the rostrocaudal axis revealed that the dopamine neurons within the medial aspect of the SNc were the most sensitive to MPTP-induced loss. Nigral neuron survival was also compared using Abercrombie's correction for estimation of neuronal populations (Abercrombie, 1946). By this method, inhibition of calpains using MDL-28170 (MDL-MPTP) significantly attenuated the overall loss of nigral TH+ neurons to 17.8% versus a 55.9% loss of nigral dopamine neurons after MPTP treatment in vehicle-treated controls (VEH-MPTP). One-way ANOVA revealed that the neuroprotection conferred by calpain inhibition did not differ from saline-treated controls at 2 weeks after MPTP (p < 0.0008; Newman-Keuls, p < 0.05) (Fig. 3d, Table 3). To evaluate whether inhibition of calpains was a transitory effect on neuron survival after MPTP treatment, we next examined the degree of nigral neuron survival 3 weeks (21 d) after MPTP in mice coadministered vehicle or the calpain inhibitor. Consistent with the neuroprotection observed at 14 d, at 21 d after MPTP significantly greater numbers of nigral dopamine neurons were still evident, as evaluated by either TH immunostaining or cresyl violet staining (data not shown), in the MPTP-lesioned mice treated with the calpain inhibitor MDL-28170 when compared with vehicle-lesioned controls (Table 3).
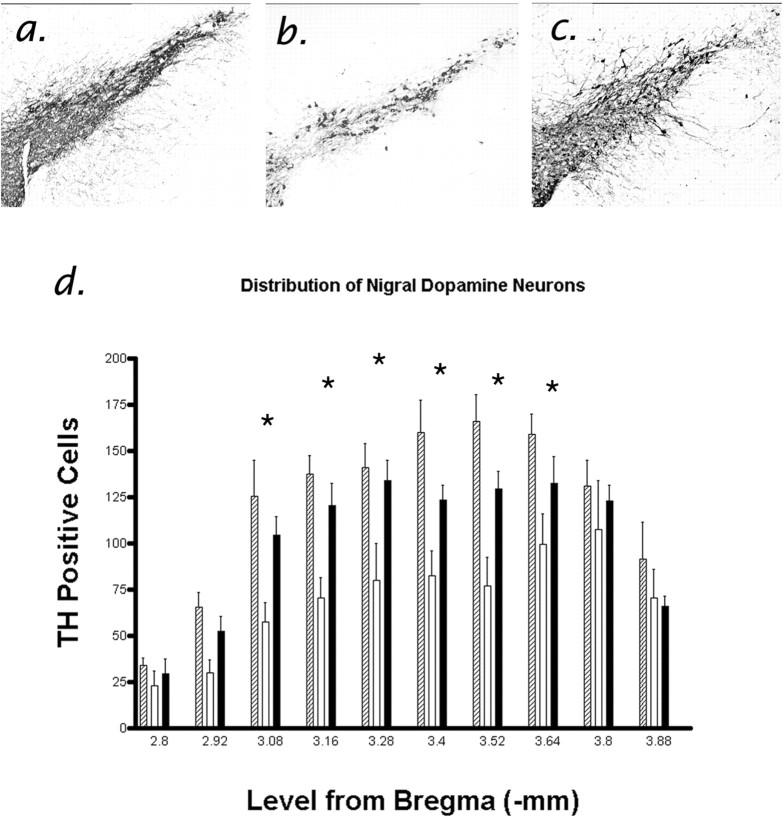
Figure 3.
Protection of nigral dopamine neurons by calpain inhibition. Representative coronal midbrain sections (at level - 3.52 mm caudal to bregma) from mice 2 weeks after treatment with saline (a), MPTP with vehicle (b), or MPTP with the calpain inhibitor MDL-28170 (c). d, Quantitative comparison of nigral dopamine (TH+) neuron survival in mice treated with saline (hatched bars), vehicle and MPTP (white bars), or MDL 28170 and MPTP (black bars). Bars represent mean number (±SEM) of neurons according to the rostrocaudal distribution measured (millimeters) from bregma (Franklin and Paxinos, 1997) from eight mice per group (ANOVA, p < 0.01; Newman-Keuls, p < 0.05).
Table 3.
Calculation of neuronal MPP + metabolism (nanograms per milligram tissue) and calculations of nigral dopamine neuron (SNc) survival in saline and MPTP treatment groups
|
|
Saline |
VEH-MPTP |
MDL-MPTP |
|
p |
|---|---|---|---|---|---|
| MPP+ | N/A | 4.566 ± 0.579 | 4.064 ± 0.644 | (t) | 0.5781 |
| 14 d TH | 12259 ± 837.8 | 6854 ± 859.5* | 10069 ± 629.6 | (A) | 0.0008 |
| 21 d TH | 14082 ± 2123.8 | 5061 ± 993.94* | 12905 ± 1789.9 | (A) | 0.0038 |
| 21 d CV
|
17086 ± 881.8
|
7366 ± 1923.2*
|
13961 ± 1164.3
|
(A)
|
0.0003
|
Estimates of nigral neuron populations were performed on tyrosine hydroxylase (TH)- or cresyl violet (CV)-stained midbrain tissue sections and calculated using Abercrombie correction. Data are presented as group mean ± SEM. Statistical differences are given as p values for either t test (t) or one-way ANOVA (A) with significance represented by asterisks.
MPTP metabolism is not affected by calpain inhibition (MDL-28170)
The neurotoxic effects of MPTP on dopamine neurons require the metabolism of MPTP to MPP+ by monoamine oxidase (Heikkila et al., 1984; Javitch et al., 1985; Przedborski et al., 2001). Hence, to determine whether pharmacological inhibition of calpains modified the metabolism of MPTP (and thus indirectly prevented nigral degeneration), we extracted striatal tissues from mice 90 min after a single injection of MPTP (30 mg/kg) and measured levels of MPP+. This analysis revealed that calpain inhibition did not influence the metabolism of MPTP to MPP+ in vivo (p > 0.28) (Table 3), indicating that the observed protection by calpain inhibition was not caused by an indirect mitigation of injury through impaired MPTP metabolism.
Calpastatin overexpression is neuroprotective
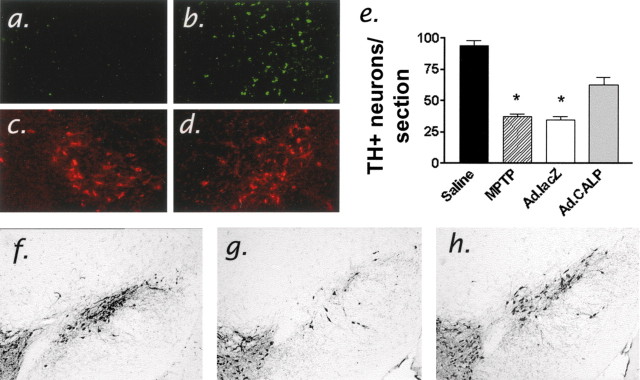
Next, we engineered a recombinant adenovirus to overexpress the endogenous calpain inhibitor protein, calpastatin, in vivo (De Tullio et al., 1998; Crocker et al., 2001a). Adenoviruses expressing either lacZ (Ad.lacZ) or calpastatin (Ad.CALP) were injected directly into the right dorsolateral striata of mice to produce retrograde transport (Fig. 4b) and expression within nigrostriatal dopamine neurons (Fig. 4d) (Ridoux et al., 1994; Crocker et al., 2001a). The degree of neuronal loss in the contralateral (non-virus injected) hemispheres of Ad.lacZ and Ad.CALP mice did not differ after MPTP treatment (t test; p > 0.84). Compared with the SNc of unlesioned mice (Fig. 4e), the number of surviving dopamine neurons in the ipsilateral hemisphere of MPTP-treated Ad.lacZ-treated mice was significantly less and comparable with the loss of dopamine neurons observed in the contralateral (non-virus injected) SNc (Fig. 4f,h). In contrast, the number of nigral neurons in the ipsilateral (adenoviral injected) SNc of Ad.CALP-treated mice was significantly greater than its contralateral hemisphere (Fig. 4g,h). Quantitative comparison indicated that adenoviral expression of calpastatin significantly attenuated MPTP-induced neuron loss, doubling the number of surviving TH+ SNc neurons (Fig. 4h). To verify that adenovirus-mediated expression of calpastatin was affecting calpain-related proteolysis after MPTP administration, we also determined that calpain-cleaved α-spectrin (38-4) immunoreactivity was significantly attenuated in the ipsilateral calpastatin-expressing SNc when compared with the control contralateral side (data not shown).
Figure 4.
Increased calpastatin in the SNc ipsilateral to intrastriatal adenovirus administration (a), when compared with the contralateral hemisphere (b), and colocalization within dopaminergic SNc neurons (c,d). Representative coronal midbrain section at the level of the MTN (-3.16 mm caudal to bregma) (Franklin and Paxinos, 1997) from a saline-treated mouse (f) and the ipsilateral hemisphere of MPTP-treated mice that received intrastriatal administration of recombinant adenoviruses containing either lacZ (g) or the calpain inhibitor protein calpastatin (h). Images are representative of dopamine neuron survival observed 2 weeks after treatment with MPTP (n = 6 per group). e, Quantification of dopamine neuron survival per section in the ipsilateral SNc of Ad.lacZ, Ad.CALP, and uninjected-MPTP-treated or unlesioned mice. *ANOVA, p < 0.0001; Newman-Keuls, p < 0.001; Ad.CALP versus Ad.lacZ or MPTP.
MPTP-induced hypolocomotion is abrogated by calpain inhibition
The ultimate therapeutic goal of neuroprotection is to ameliorate functional impairment. Accordingly, we next determined whether the protection of nigral dopamine neurons by inhibition of calpain-mediated proteolysis was also accompanied by an absence of locomotor deficit, one important hallmark of PD. Using a video-tracking system, we evaluated the horizontal locomotor activity of mice in a novel environment. In this setting, MPTP-treated mice displayed a modest 30% decrease in spontaneous motor activity when compared with either group of non-MPTP-treated control animals (Fig. 5a). In contrast, mice that received the calpain inhibitor MDL-28170 exhibited significantly more activity than MPTP-lesioned controls (p < 0.01) and did not differ in total spontaneous activity from unlesioned controls (Fig. 5a(p > 0.05). The deficit in spontaneous locomotor activity observed in MPTP lesioned mice in this open field test was also reversed by administration of l-Dopa (15 mg/kg, i.p.) at the time of testing (Newman-Keuls; p > 0.05). Thus, MPTP treatment produced a significant reduction spontaneous locomotor performance that was prevented by inhibition of calpains (ANOVA, p < 0.008; p > 0.05 vs saline VEH-MPTP + l-Dopa). Mice treated with calpain inhibitor but without MPTP did not differ from saline-treated mice (data not shown).
Figure 5.
The neuroprotective effects of calpain inhibition on MPTP-induced toxicity in mice is associated with normalized locomotor behaviors. Two weeks after saline or MPTP treatment, groups of mice were assessed for spontaneous motor activity in a novel environment (open field) for a 60 min period, as described in Materials and Methods (a). Horizontal locomotor activity was reported as distance traveled (mean ± SEM; *ANOVA, p < 0.001; Newman-Keuls, p < 0.01; either group vs VEH-MPTP). Additional groups of mice were administered amphetamine(2.0 mg/kg, i.p.), and hyperactivity was measured in their home cages using beam-break activity monitors (b). Total activity over 30 min is plotted as mean ± SEM. Data represent n = 6-9 per group per treatment for a and b.
Next we examined whether the improved activity in calpain-treated mice was a consequence of enhanced dopamine-related neurotransmission. To test this, additional groups of mice were treated with saline, MPTP and vehicle, or MPTP and MDL-28170. These groups were kept in their home cages to minimize any influence of natural exploratory behaviors observed in a novel environment. These cohorts were challenged with amphetamine (2.0 mg/kg, i.p.) to stimulate the release of dopamine from intact nerve terminals. In this experiment, amphetamine-induced hyperlocomotor activity was used as a functional measure of dopamine-related function within the basal ganglia. Amphetamine-induced locomotor responses were severely impaired in MPTP-treated mice that were infused with the vehicle solution (Fig. 5b, VEH-MPTP), whereas MPTP-treated mice that were cotreated with calpain inhibitor displayed robust activity in response to amphetamine challenge (MDL-MPTP) and exhibited a level of activity equal to saline-treated control animals (Fig. 5b). Taken together, these results indicate that calpain inhibition prevented impaired locomotor performance in response to either a novel environment or an amphetamine challenge. Interestingly, there is some variation in the literature regarding the presence and severity of motor behavior deficits in the MPTP mouse model, perhaps because of subtle differences in mouse stains, dosing regimens, or modes of analyses (Sedelis et al., 2001). However, our findings are consistent with the growing majority of studies reporting hypolocomotion.
Calpain inhibition and striatal denervation
Because calpain inhibition prevented the detrimental effects of MPTP on locomotor performance, we next evaluated the status of nigrostriatal function in groups of mice by assessing the expression of various neurochemical and immunohistochemical markers for dopaminergic neurotransmission. Immunohistochemical detection of TH fiber staining in striatal tissue sections from saline-treated (Fig. 6a), VEH-MPTP-treated (Fig. 6b), or MDL-MPTP-treated (Fig. 6c) mice revealed that MPTP-induced depletion of nigrostriatal dopaminergic fibers was only slightly mitigated by treatment with the calpain inhibitor. Densitometric analysis of striatal TH staining revealed a significant loss of TH fibers in VEH-MPTP-treated mice and a lessened but significant loss in mice treated with calpain inhibitor (Fig. 6g). Although calpain inhibition partially ameliorated the degree of loss of TH fibrous staining, both groups of MPTP-treated mice were significantly different from saline-treated controls. Finally, adjacent tissues sections assessed for expression of DAT revealed a similar degree of dopamine terminal loss as that detected by TH immunostaining in these groups (Fig. 6d-f).
Figure 6.
Calpain inhibition does not prevent striatal denervation. Representative immunohistochemical detection of tyrosine hydroxylase (TH) (a-c) and dopamine transporter (DAT) (d-f) in striatal sections from mice treated with saline (a, d), MPTP, and vehicle (b, e), or MDL-28170 and MPTP (c, f). g, Quantification of striatal TH fiber densities (ANOVA, p < 0.001; Newman-Keuls, **p < 0.001, and *p < 0.05, vs saline).
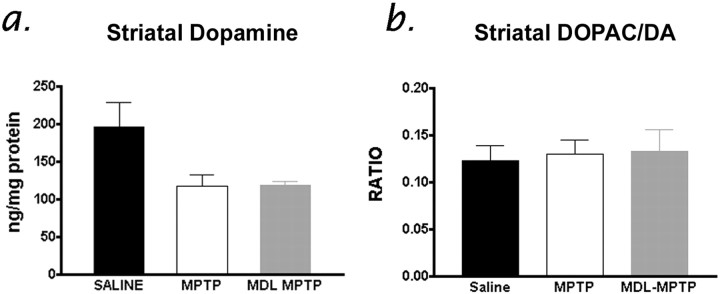
To establish whether the modest DA terminal protection offered by calpain inhibition correlated with dopamine levels in the striatum, HPLC detection of DA and metabolites was performed on tissues VEH-MPTP, MDL-MPTP, and saline-treated mice. This analysis revealed that MPTP administration caused a significant 60.25% (average) drop in striatal dopamine levels in all MPTP-treatment groups, including those receiving the calpain inhibitor (Fig. 7a) (p < 0.0002). Analyses of the dopamine metabolites, DOPAC and homovanillic acid, did not indicate any compensatory increase in dopamine turnover (Fig. 7b), which has been reported by some (Vila et al., 2001) but not by others (Yang et al., 1998; Eberhardt et al., 2000). Nevertheless, in this study the absence of protection or compensation of dopamine levels in the striata of calpain inhibitor-treated mice therefore cannot account for the improved behavioral performance in this treatment group (Fig. 7b).
Figure 7.
HPLC analysis of striatal dopamine (a) and DOPAC concentrations (b) shows no preservation of striatal dopamine or metabolism after MPTP administration in either vehicle (VEH-MPTP) or calpain inhibitor-treated (MDL-MPTP) mice. Data represent mean ± SEM (n = 6-8 per group). Where no significant differences existed between VEH-MPTP and MPTP treatment groups, data were pooled as “MPTP.”
Calpain inhibition prevents postsynaptic changes in gene expression in the striatum
Because calpain inhibition prevented the loss of motor function in MPTP lesioned mice but did not prevent the loss of striatal dopaminergic fibers, we surmised that one possible consequence of nigral neuroprotection by calpain inhibition might have been the indirect modulation of postsynaptic changes in the denervated striatum. To analyze for this possibility, we looked for changes in postsynaptic markers of denervation in the striatum of mice. The first marker of neuronal plasticity that is upregulated substantially in the striatum in response to dopaminergic denervation is the immediate early gene, FosB (Doucet et al., 1996; Perez-Otano et al., 1998). FosB has garnered attention recently for its roles in the processes mediating the neural plasticity associated with addiction (Nestler et al., 1999) and as a causal signal mediating the supersensitivity of striatal dopamine receptors after dopaminergic denervation (Crocker et al., 1998; Andersson et al., 1999). Striatal tissue sections from mice treated with saline, MPTP, VEH-MPTP, or MDL-MPTP were processed for immunohistochemical detection of FosB. Consistent with previous reports (Doucet et al., 1996; Perez-Otano et al., 1998), MPTP induced a profound increase in FosB expression in the striatum (Fig. 8a-c). However, coadministration of MDL-28170 significantly attenuated the MPTP-induced increase in striatal FosB expression (Fig. 8d). Quantification of striatal FosB nuclei showed that mice lesioned with MPTP and treated with the calpain inhibitor expressed significantly fewer FosB-like immunoreactive neurons than other MPTP-lesioned animals (Fig. 8e). Interestingly, FosB expression in MDL-MPTP-treated mice did not differ from saline-treated controls (p > 0.05).
Figure 8.

MPTP-induced increased striatal FosB expression is attenuated by calpain administration. Representative coronal forebrain sections depict low basal expression of FosB in the striatum (a) and a dramatic elevation 2 weeks after chronic administration of MPTP (b). Striatal sections from mice administered MPTP and vehicle revealed a comparable upregulation of FosB (c), whereas tissues from mice administered MPTP and the calpain inhibitor MDL-28170 revealed an attenuation of postsynaptic FosB expression (d). e, Quantification of striatal FosB-positive nuclei show significant differences between treatment groups (*ANOVA, p < 0.0005; Newman-Keuls, p < 0.01 compared with saline control). FosB expression in MDL-MPTP-treated mice did not differ from saline-treated controls (p > 0.05). Data represent mean ± SEM (n = 5-6 per group).
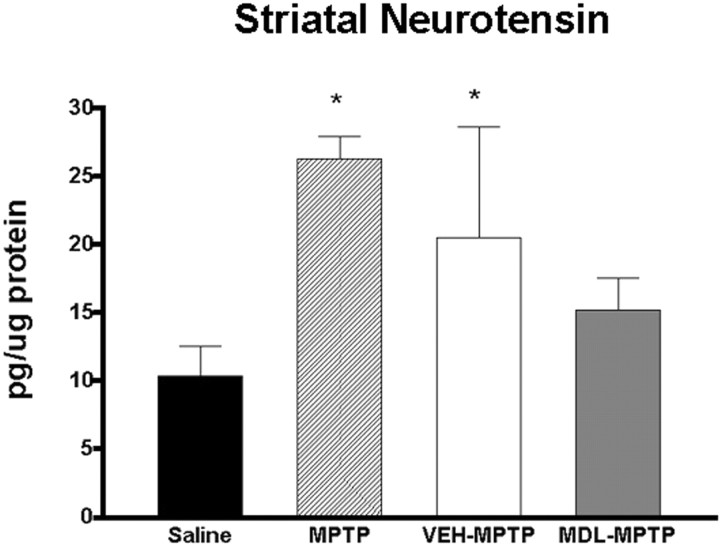
The role of neuropeptides as modulators of basal ganglia activity has led to the suggestion that these molecules may contribute to dysfunction related to PD pathology. As a second marker of postsynaptic changes resulting from dopaminergic denervation, we looked at NT because recent reports suggest that endogenous NT may antagonize dopamine neurotransmission (Ford and Marsden, 1990; Radke et al., 1998; Binder et al., 2001), and striatal expression of NT has been reported to increase after MPTP administration to mice (Martorana et al., 2001) and in postmortem human PD tissues (Fernandez et al., 1995; Schimpff et al., 2001). Given the possible modulatory effects that NT may exert on basal ganglia function, we therefore determined whether MPTP-induced changes in neurotensin were modified by administration of the calpain inhibitor MDL-28170. Radioimmunoassay of striatal tissues from saline- and MPTP-treated groups of mice revealed a 2.5-fold increase in neurotensin levels in MPTP-treated mice 2 weeks after cessation of treatment, when compared with saline-treated controls (Fig. 9). In contrast, MPTP mice treated with the calpain inhibitor did not exhibit any changes in striatal NT expression (Fig. 9), which suggests that calpain inhibition prevented postsynaptic changes in markers of gene and neuropeptide changes in the dopaminergic denervated striatum.
Figure 9.
Radioimmunoassay of striatal neurotensin revealed increased expression after treatment with MPTP that was attenuated by calpain inhibitor treatment (*ANOVA; p < 0.05). Data represent mean ± SEM (n = 7-8 per group).
Evidence for calpain activation in postmortem human Parkinson's disease tissues
Finally, we examined whether calpain activation participates in the human PD condition, as observed in the MPTP-treated mice. Tissues from the midbrains of individual cases with premortem diagnosis of either Parkinson's disease or no known neurological condition were analyzed for immunohistochemical detection of calpain activity using the calpain-cleaved α-spectrin antibody (Tables 1, 2). Qualitatively, the distribution of calpain-cleaved α-spectrin in nigral neurons of control cases was weak and diffusely cytoplasmic, whereas tissues from PD subjects exhibited an intense speckled appearance that resembled the staining observed in the nigral dopamine neurons of MPTP-treated mice (Figs. 10 and 2, respectively). The proportion of pigmented SNc neurons immunopositive for calpain-cleaved α-spectrin was significantly higher in PD cases (52.83%) when compared with expression in control subjects (9.2%; Mann-Whitney; p = 0.0079) (Fig. 10D). These data provide a potentially interesting and novel correlation of the human PD condition with changes observed in MPTP-treated mice.
Figure 10.
Immunohistochemical evidence of enhanced calpain activity in nigral neurons in postmortem human tissues. A, Immunohistochemical detection of calpain-cleaved α-spectrin (38-4) viewed at low (20×) magnification revealed intense clusters of immunopositive staining in PD cases that were rarely observed in control cases. B, C, Confocal optical sectioning (0.6 μm) of calpain-cleaved α-spectrin immunoreactivity in pigmented dopamine neurons from a case without neurological condition (B) and from a case with Parkinson's disease (C) further revealed the cytoplasmic speckled appearance of 38-4-immunopositive staining that colocalized with neuromelanin (insets). D, Quantification of the proportion of pigmented neurons per section that displayed speckled calpain-cleavedα-spectrin immunoreactivity from PD and control tissues revealed a significant increase in calpain activity in subjects with PD (**52.83 ± 7.5 vs 9.17 ± 3.1%; Mann-Whitney rank-sum test; p = 0.0079). Positive immunostaining disappeared when the primary antibody was omitted (data not shown). Scale bars: A,25μm; B, C,50μm.
Discussion
Although calpains mediate various cellular functions, the results of this study support the hypothesis that inappropriate or sustained activation of calpain-related proteolysis may precipitate neuropathology (Saito et al., 1993; Roberts-Lewis et al., 1994; Mouatt-Prigent et al., 1996; Markgraf et al., 1998; Lee et al., 2000). In this regard, it is important to note that calpains are calcium dependent, indicating that processes involving the management of intracellular calcium can influence the state of calpain activation. Maintenance of calcium homeostasis is critical for neuronal viability, necessitating tight regulation of its intracellular concentrations. In neurons, mitochondria are a central store of calcium as well as the primary source of cellular oxidative metabolism. The finding that dopaminergic neurotoxins such as MPTP (MPP+) are also mitochondrial toxins has led to the hypothesis that impaired mitochondrial function may underlie the nigral pathogenesis of PD. Indeed, deficits in complex 1 activity in PD patients (Greenamyre et al., 2001) and evidence for increased oxidative stress in nigral neurons have been reported (for review, see Przedborski and Jackson-Lewis, 2000). In addition, mitochondria are an integral participant in signaling pathways of apoptotic neuronal death, which has been proposed to mediate neuronal loss in PD (Cassarino et al., 1999; Hartmann et al., 2000; Mochizuki et al., 2001; Vila et al., 2001; Viswanath et al., 2001).
Several lines of evidence support the hypothesis of calcium-mediated pathology in PD: (1) MPP+ evokes sustained increases of intracellular calcium in neurons (Frei and Richter, 1986; Chen et al., 1995; Sherer et al., 2001); (2) mitochondria from PD patients exhibit a diminished capacity to sequester calcium (Sheehan et al., 1997); (3) nigral neurons that express the calcium-binding protein, calbindin D, are less vulnerable to degeneration in PD, perhaps because of the ability to buffer increased levels of intracellular calcium (Yamada et al., 1990; Lavoie and Parent, 1991; Hirsch et al., 1997; McMahon et al., 1998; Damier et al., 1999); (4) calcium-regulating genes, such as nitric oxide synthase, have been implicated in dopamine neuron degeneration in PD (Schulz et al., 1995; Liberatore et al., 1999); and (5) energy disruption alone is insufficient to explain the toxicity of MPP+ (Nakamura et al., 2000). It is important to note that although the sustained calcium-mediated activation of calpains in mice in our study is likely a consequence of the mitochondrial toxicity of MPTP, in the human cases of PD, calcium may arise from mitochondria or other possible sites. For instance, mitochondrial dysfunction results in a paucity of energy (ATP) that could evoke release from endoplasmic reticular stores (Mattson et al., 2000; Paschen and Frandsen, 2001) or modulate influx of calcium from extracellular sources (Greenamyre et al., 1999). Finally, we cannot rule out the possibility that calpain regulation may occur through mechanisms other than an abberant calcium response. This is important because the physiological regulation of calpains is complex, involving numerous factors including calcium, phosphorylation, and nitrosylation (Sato and Kawashima, 2001; Shiraha et al., 2002; Forsythe and Befus, 2003). Although our results validate a role for calpains in PD, the identity of the essential calpain species involved in the loss of nigral dopamine neurons after MPTP is also unresolved. It is important to note that although previous work on postmortem human tissues has reported increased expression of m-calpain in PD, it not presently clear whether MPTP also recapitulates this selectivity of calpain involvement in mice.
The manner by which calpains mediate dopaminergic death remains to be elucidated. Putative calpain substrates that have been associated with neurodegeneration in PD include c-Jun (Hirai et al., 1991; Saporito et al., 1999; Xia et al., 2001) and p53 (Trimmer et al., 1996; Gonen et al., 1997). In general, the short half-life of calpain substrates coupled with the restricted nature of calpain cleavage of these target proteins has led to the suggestion that calpains act to modify rather than completely catabolize substrate proteins (Suzuki et al., 1992; Carafoli and Molinari, 1998). For instance, the transcriptional activity of c-Jun is significantly reduced in the presence of activated calpain, suggesting that calpains may influence signal transduction by modulating gene expression (Suzuki et al., 1992). In addition, the cyclin-dependent kinase 5 (cdk5) activating protein p35 has been reported to be cleaved by calpains into a p25 isoform that results in unregulated cdk5 activity and neurodegeneration (Patrick et al., 1999; Lee et al., 2000). Hence, various potential calpain substrates may directly or indirectly contribute to calpain-related degeneration of dopamine neurons.
Nigral management of basal ganglia function
A second aspect of this study pertinent to the understanding of PD is that calpain inhibition conferred nigral neuroprotection without replenishment of striatal dopamine yet prevented deficits in locomotor behavior in MPTP-treated mice and normalized markers of BG activity. Historically, the symptoms of Parkinson's disease have been considered to be a consequence of diminished striatal dopamine. Indeed, dopamine replacement therapies have supported this concept. However, previous work has also suggested that the actions of l-Dopa may not be restricted to surviving dopamine terminals in the striatum, but the substantia nigra may too be a significant site for the actions of l-Dopa and dopaminergic regulation of movement (Robertson and Robertson, 1988, 1989; Crocker, 1997; Fox et al., 1998).
It has long been recognized that nigral dopaminergic neurons release dopamine not only from their axons projecting to the striatum but also from their dendrites (Bjorklund and Lindvall, 1975; Cheramy et al., 1981). Therefore dendro-dendritic release of dopamine by SNc neurons may be of particular importance in terms of the function of the BG in PD (Robertson and Robertson, 1989; Tseng et al., 1997; Fox et al., 1998; Gainetdinov et al., 1999; Collier et al., 2002). Results of this study present evidence to suggest that preservation of nigral integrity may provide preservation of dopaminergic-related motor function after loss of striatal nerve terminals. Although the precise nature of the functional benefit associated with nigral neuroprotection by calpain inhibition is presently not known, there are several possibilities pertaining to nigral dopaminergic regulation of BG function.
The first possibility involves the counterbalancing influences of serotoninergic inputs to the substantia nigra pars reticulata (SNr). There is increasing evidence that dopaminergic denervation after degeneration of nigrostriatal neurons results in increased serotoninergic innervation of the reticulostriatal pathway (Thibaut et al., 1995; Fox and Brotchie, 2000). Interestingly, this influence can be attenuated by direct administration of 5HT2C receptor antagonists into the SN (Fox et al., 1998). The function of serotonin in this context is thought to exacerbate hypolocomotor activity. This notion is exemplified in DAT-deficient mice in which basal hyperactivity can be modulated by enhancing serotonergic activity, without affecting levels of striatal dopamine (Gainetdinov et al., 1999). In the present study, the prevention of SNc degeneration by calpain inhibition may have preserved the integrity of dopaminergic innervation of the SNr and therein acted to circumvent a serotonin-mediated hypolocomotion.
A second possibility relates to dopaminergic modulation of excitatory innervation of the SNr. Dopaminergic innervation of the SNr is thought to modulate the activation states of glutamate receptors on excitatory synapses from the subthalamic nucleus (Wittmann et al., 2002). Thus, it has been proposed that SNr function, and hence BG output, may be succinctly modulated by nigral interactions of dopamine, glutamate, and GABA signaling (Kelly et al., 1987; Abarca et al., 1995; Matuszewich and Yamamoto, 1999; Wittmann et al., 2002). Under the circumstances of the present study, the preservation of the tonic innervation of the SNr by dendritic dopamine release may compensate for depleted striatal dopamine by modulating BG function.
Third, recent findings have also lead to the suggestion that extrastriatal dopamine may modulate striatonigral feedback pathways. Interestingly, intranigral infusion of glial cell line-derived neurotrophic factor in unilateral dopaminergic lesion rat models have been previously reported to reverse postsynaptic neuropeptide changes in the striatum, coincident with improved behaviors and without altering striatal dopamine concentrations (Lapchak et al., 1997; Tseng et al., 1997). These findings are also consistent with our present results. Taken together, this evidence suggests a role for extrastriatal modulation of BG-mediated motor behavior perhaps through nigral modulation of striatonigral projections (Collier et al., 2002).
Although calpain inhibition may be of benefit for the aspects of PD/MPTP-related behavior that were measured in this study, it is important to emphasize that other PD-related symptomology has not been examined and may not be improved by calpain inhibition alone. Interestingly, however, several previous studies have also reported significant protection of the SNc without preservation of striatal dopamine (Liberatore et al., 1999; Mandir et al., 1999; Vila et al., 2001). Because motor performance was not assayed in these studies, it is unclear whether protection of the SNc after MPTP by various strategies will prevent behavioral deficits or whether preservation of dopamine-related motor behaviors are selective to calpain inhibition. Nevertheless, these findings suggest that the complex circuitry of the basal ganglia likely maintains functionally redundant projections that can substitute for an absence of striatal dopamine, therein supporting our contention that the SNc may be a critical locus for dopamine-related functions in PD.
The present findings support a preeminent role for calpain-mediated proteolysis in neurodegeneration and impairment of dopaminergic functions in a model of Parkinson's disease. These findings provide evidence to support the central role of the SNc in PD and imply that motor dysfunction in PD may not be the singular result of a loss of striatal dopamine but instead may represent the manifestation of an imbalance among multiple integrated pathways of basal ganglia nuclei.
Footnotes
The Harvard Brain Tissue Resource Center is supported in part by Public Health Service Grant MH/NS 31862. This work was supported by grants from the Canadian Institutes of Health Research (CIHR)(D.S.P., Z.M., H.A), Parkinson's Society Canada (D.S.P), Parkinson's Disease Foundation (USA) (S.P., V.J.-L., D.S.P), National Institutes of Health/National Institute of Neurological Disorders and Stroke (Grants R29 NS37345, R01 NS38586 -NS 42269, P50 NS38370), United States Department of National Defense (DAMD 17-99-1-9471 to S.P.), Lowenstein Foundation, Lillian Goldman Charitable Trust, Muscular Dystrophy Association, ALS Association (S.P.), and National Parkinson's Foundation (V.J.-L.). W.R.L. was supported by a summer studentship from the Parkinson's Disease Foundation (USA). S.J.C. was funded by a postdoctoral fellowship from the Ontario Neurotrauma Foundation. H.A. is a Canada Research Chair in Neuroscience. D.S.P. is a recipient of the Glaxo-Wellcome Chair for Stroke and is a CIHR Scholar. We thank Dr. R. Simon for helpful discussions. We also thank Dr. A. Ridsdale for assistance with confocal microscopy, and Z. Kulczycki, N. Lukenbill, and K. Yates for technical assistance. We gratefully acknowledge Cephalon Inc. (West Chester, PA) for antibodies (Roberts-Lewis et al., 1994).
Correspondence should be addressed to Dr. David S. Park, 451 Smyth Road, Ottawa, Ontario, Canada K1H 8M5. E-mail: dpark@uottawa.ca.
Copyright © 2003 Society for Neuroscience 0270-6474/03/234081-11$15.00/0
References
- Abarca J, Gysling K, Roth RH, Bustos G ( 1995) Changes in extracellular levels of glutamate and aspartate in rat substantia nigra induced by dopamine receptor ligands: in vivo microdialysis studies. Neurochem Res 20: 159–169. [DOI] [PubMed] [Google Scholar]
- Abercrombie M ( 1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247. [DOI] [PubMed] [Google Scholar]
- Andersson M, Hilbertson A, Cenci MA ( 1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis 6: 461–474. [DOI] [PubMed] [Google Scholar]
- Arthur JSC, Elce JS, Hegadorn C, Williams K, Greer PA ( 2000) Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol 20: 4474–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF ( 2001) Experimental models of Parkinson's disease. Nat Rev Neurosci 2: 325–334. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB ( 2001) Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci 21: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O ( 1975) Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res 83: 531–537. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Molinari M ( 1998) Calpain: a protease in search of a function? Biochem Biophys Res Commun 247: 193–203. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker Jr WD, Bennett Jr JP ( 1999) The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta 1453: 49–62. [DOI] [PubMed] [Google Scholar]
- Chen TS, Koutsilieri E, Rausch WD ( 1995) MPP+ selectively affects calcium homeostasis in mesencephalic cell cultures from embryonal C57/Bl6 mice. J Neural Transm Gen Sect 100: 153–163. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Leviel V, Glowinski J ( 1981) Dendritic release of dopamine in the substantia nigra. Nature 289: 537–542. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE, Elsworth JD, Taylor JR, Roth RH, Sladek Jr JR, Redmond Jr DE ( 2002) Embryonic ventral mesencephalic grafts to the substantia nigra of MPTP-treated monkeys: feasibility relevant to multiple-target grafting as a therapy for Parkinson's disease. J Comp Neurol 442: 320–330. [DOI] [PubMed] [Google Scholar]
- Crocker AD ( 1997) The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Rev Neurosci 8: 55–76. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Morelli M, Wigle N, Nakabeppu Y, Robertson GS ( 1998) D1-receptor-related priming is attenuated by antisense-meditated “knock-down” of fosB expression. Brain Res Mol Brain Res 53: 69–77. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Lamba WR, Smith PD, Callaghan SM, Slack RS, Anisman H, Park DS ( 2001a) c-Jun mediates axotomy-induced dopamine neuron death in vivo. Proc Natl Acad Sci USA 98: 13385–13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Wigle N, Liston P, Thomspon CS, Lee CJ, Xu DG, Roy S, Nicholson DW, Park DS, MacKenzie AE, Korneluk RG, Robertson GS ( 2001b) NAIP protects nigrostriatal dopamine pathway in an intrastriatal 6-OHDA rat model of Parkinson's disease. Eur J Neurosci 14: 391–400. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM ( 1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437–1448. [DOI] [PubMed] [Google Scholar]
- De Tullio R, Sparatore B, Salamino F, Melloni E, Pontremoli S ( 1998) Rat brain contains multiple mRNAs for calpastatin. FEBS Lett 422: 113–117. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS ( 1996) Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of delta-FosB-like protein(s) in both rodent and primate striatum. Eur J Neurosci 8: 365–381. [DOI] [PubMed] [Google Scholar]
- Eberhardt O, Coelin RV, Kugler S, Lindeneau J, Rathke-Hartlieb S, Gerhardt E, Haid S, Isenman S, Gravel C, Srinivasen A, Bahr M, Weller M, Dichgans J, Schulz JB ( 2000) Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci 20: 9126–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y, Kawasaki H, Imajoh S, Imajoh K, Suzuki K ( 1987) Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc Natl Acad Sci USA 84: 3590 -3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Jenner P, Marsden CD, De Ceballos ML ( 1995) Characterization of neurotensin-like immunoreactivity in human basal ganglia: increased neurotensin levels in substantia nigra in Parkinson's disease. Peptides 16: 339–346. [DOI] [PubMed] [Google Scholar]
- Ford APDW, Marsden CA ( 1990) In vivo neurochemical and behavioural effects of intracerebrally administered neurotensin and d-Trp11-neurotensin on mesolimbic and nigrostriatal dopaminergic function in the rat. Brain Res 53: 243–250. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Befus AD ( 2003) Inhibition of calpains is a component of nitric oxide-induced induced down-regulation of human mast cell adhesion. J Immunol 170: 287–293. [DOI] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM ( 2000) 5-HT2C receptor binding is increased in the substantia nigra pars reticulata in Parkinson's disease. Mov Disord 15: 1064–1069. [DOI] [PubMed] [Google Scholar]
- Fox SH, Moser B, Brotchie JM ( 1998) Behavioral effects of 5-HT2C receptor [DOI] [PubMed]
- Franklin KBJ, Paxinos G ( 1997) The mouse brain in stereotaxic coordinates. Toronto: Academic.
- Frei B, Richter C ( 1986) N-methyl-4-phenylpyridine (MPP+) together with 6-hydroxydopamine of dopamine stimulates Ca 2+ release from mitochondria. FEBS Lett 198: 99–102. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG ( 1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283: 397–401. [DOI] [PubMed] [Google Scholar]
- Gonen H, Shkedy D, Barnoy S, Kosower NS, Ciechanover A ( 1997) On the involvement of calpains in the degradation of the tumor suppressor protein p53. FEBS Lett 406: 17–22. [DOI] [PubMed] [Google Scholar]
- Goto K, Iwamoto T, Kondo H ( 1994) Localization of mRNAs for calpain and calpastatin in the adult rat brain by in situ hybridization histochemistry. Brain Res Mol Brain Res 23: 40–46. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, MacKenzie G, Peng TI, Stephans SE ( 1999) Mitochondrial dysfunction in Parkinson's disease. Biochem Soc Symp 66: 85–97. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Sherer TB, Betarbet R, Panov AV ( 2001) Complex I and Parkinson's disease. IUBMB Life 52: 135–141. [DOI] [PubMed] [Google Scholar]
- Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML ( 1997) Construction of adenovirus vectors through Cre-lox recombination. J Virol 71: 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC ( 2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA 97: 2875–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Troadec JD, Hunot S, Kikly K, Faucheux BA, Mouatt-Prigent A, Ruberg M, Agid Y, Hirsch EC ( 2001) Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease but pathway inhibition results in neuronal necrosis. J Neurosci 21: 2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Brebner K, Lacosta S, Merali Z, Anisman H ( 1999) Sensitization to the effects of tumor necrosis factor-α: neuroendocrine, central monoamine, and behavioral variations. J Neurosci 19: 5654–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC ( 1984) Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature 311: 467–469. [DOI] [PubMed] [Google Scholar]
- Hirai S, Kawasaki H, Yaniv M, Suzuki K ( 1991) Degradation of transcription factors, c-Jun and c-Fos, by calpain. FEBS Lett 287: 57–61. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA ( 1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature 334: 345–348. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y ( 1997) Neuronal vulnerability in Parkinson's disease. J Neural Transm [Suppl] 50: 79–88. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH ( 1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA 82: 2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P ( 1998) Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov Disord 13[Suppl 1]: 24–34. [PubMed] [Google Scholar]
- Kass GE, Wright JM, Nicotera P, Orrenius S ( 1988) The mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity: role of intracellular calcium. Arch Biochem Biophys 260: 789–797. [DOI] [PubMed] [Google Scholar]
- Kelly E, Jenner P, Marsden CD ( 1987) Comparison of changes in locomotor activity with striatal homovanillic acid and 3,4-dihydroxyphenylacetic acid concentrations following the bilateral intranigral injection of dopamine agonist drugs in rats. J Pharm Pharmacol 39: 196–202. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Miller PJ, Collins F, Jiao S ( 1997) Glial cell line-derived neurotrophic factor attenuates behavioural deficits and regulates nigrostriatal dopaminergic and peptidergic markers in 6-hydroxydopamine-lesioned adult rats: comparison of intraventricular and intranigral delivery. Neuroscience 78: 61–72. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A ( 1991) Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. NeuroReport 2: 601–604. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH ( 2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364. [DOI] [PubMed] [Google Scholar]
- Li J, Grynspan F, Berman S, Nixon R, Bursztajn S ( 1996) Regional Differences in gene expression for calcium activated neutral proteases (calpains) and their endogenous inhibitor calpastatin in mouse brain and spinal cord. J Neurobiol 30: 177–191. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S ( 1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM ( 1999) Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci USA 96: 5774–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf CG, Velayo NL, Johnson MP, McCarthy DR, Medhi S, Koehl JR, Chmielewski PA, Linnik MD ( 1998) Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke 29: 152–158. [DOI] [PubMed] [Google Scholar]
- Martorana A, Fusco FR, Picconi B, Massa R, Bernardi G, Sancesario G ( 2001) Dopamine denervation induces neurotensin immunoreactivity in GABA-parvalbumin striatal neurons. Synapse 41: 360–362. [DOI] [PubMed] [Google Scholar]
- Mattson MP, LaFeria FM, Chan SL, Leissring MA, Shepel PN, Geiger JD ( 2000) Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 23: 222–229. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK ( 1999) Modulation of GABA release by dopamine in the substantia nigra. Synapse 32: 29–36. [DOI] [PubMed] [Google Scholar]
- McMahon A, Wong BS, Iacopino AM, Ng MC, Chi S, German DC ( 1998) Calbindin-D28k buffers intracellular calcium and promotes resistance to degeneration in PC12 cells. Brain Res Mol Brain Res 54: 56–63. [DOI] [PubMed] [Google Scholar]
- Melloni E, De Tullio R, Averna M, Tedesco I, Salamino F, Sparatore B, Pontremoli S ( 1998) Properties of calpastatin forms in rat brain. FEBS Lett 431: 55–58. [DOI] [PubMed] [Google Scholar]
- Merali Z, Piggins H ( 1990) Effects of dopamine D1 and D2 receptor agonists and antagonists on bombesin-induced behaviours. Eur J Pharmacol 191: 281–293. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Hayakawa H, Migita M, Shibata M, Tanaka R, Suzuki A, Shimo-Nakanishi Y, Urabe T, Yamada M, Tamayose K, Shimada T, Miura M, Mizuno Y ( 2001) An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA 98: 10918–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC ( 1996) Increased M-calpain expression in the mesencephalon of patients with Parkinson's disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience 73: 979–987. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ ( 2000) The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol 58: 271–278. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J ( 1999) DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res 835: 10–17. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ ( 1988) Maps and guide to microdissection of the rat brain. New York: Elsevier.
- Paschen W, Frandsen A ( 2001) Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem 79: 719–725. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH ( 1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Mandelzys A, Morgan JI ( 1998) MPTP-parkinsonism is accompanied by persistent expression of a delta-FosB-like protein in dopaminergic pathways. Brain Res Mol Brain Res 53: 41–52. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V ( 2000) ROS and Parkinson's disease: a view to a kill. In: Free radicals in brain pathophysiology (Poli G, Cadenas E, Packer L, eds), pp 273–290. New York: Marcel Dekker.
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM ( 1996) Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA 93: 4565–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M ( 2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76: 1265–1274. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Vila M, Wu du C, Teismann P, Tieu K, Choi DK, Cohen O ( 2003) Free radical and nitric oxide toxicity in Parkinson's disease. Adv Neurol 91: 83–94. [PubMed] [Google Scholar]
- Radke JM, Owens MJ, Ritchie JC, Nemeroff CB ( 1998) Atypical antipsychotic drugs selectively increase neurotensin efflux in dopamine terminal regions. Proc Natl Acad Sci USA 95: 11462–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridoux V, Robert JJ, Zhang X, Perricaudet M, Mallet J, La Gal La Salle G ( 1994) Adenoviral vectors as functional retrograde neuronal tracers. Brain Res 648: 171–175. [DOI] [PubMed] [Google Scholar]
- Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, Siman R ( 1994) Immunolocalization of calpain 1-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 14: 3934–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA ( 1988) Evidence that the substantia nigra is a site of action for l-DOPA. Neurosci Lett 89: 204–208. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA ( 1989) Evidence that l-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci 9: 3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA ( 1993) Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA 90: 2628–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, Brown EM, Miller MS, Carswell S ( 1999) CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo J Pharmacol Exp Ther 288: 421–427. [PubMed] [Google Scholar]
- Sato K, Kawashima S ( 2001) Calpain function in the modulation of signal transduction molecules. Biol Chem 382: 743–751. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD ( 1989) Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1: 1269. [DOI] [PubMed] [Google Scholar]
- Schimpff RM, Avard C, Fenelon G, Lhiaubet AM, Tenneze L, Vidailhet M, Rostene W ( 2001) Increased plasma neurotensin concentrations in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 70: 784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Beal MF ( 1995) Role of nitric oxide in neurodegenerative diseases. Curr Opin Neurol 8: 480–486. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP ( 2001) Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res 125: 109–125. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB ( 1997) Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson's disease. J Neurochem 68: 1221–1233. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Trimmer PA, Borland K, Parks JK, Bennett JP Jr, Tuttle JB ( 2001) Chronic reduction in complex I function alters calcium signaling in SH-SY5Y neuroblastoma cells. Brain Res 891: 94–105. [DOI] [PubMed] [Google Scholar]
- Shiraha H, Glading A, Chou J, Jia Z, Wells A ( 2002) Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol 22: 2716–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi H, Ishiura S, Suzuki K ( 1997) Structure and physiological function of calpains. Biochem J 328: 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Saido TC, Hirai S ( 1992) Modulation of cellular signals by calpain. Ann NY Acad Sci 674: 218–227. [DOI] [PubMed] [Google Scholar]
- Tatton NA, Kish SJ ( 1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 77: 1037–1048. [DOI] [PubMed] [Google Scholar]
- Thibaut F, Faucheux BA, Marquez J, Villares J, Menard JF, Agid Y, Hirsch EC ( 1995) Regional distribution of monoamine vesicular uptake sites in the mesencephalon of control subjects and patients with Parkinson's disease: a postmortem study using tritiated tetrabenazine. Brain Res 692: 233–243. [DOI] [PubMed] [Google Scholar]
- Trimmer PA, Smith TS, Jung AB, Bennett JP Jr ( 1996) Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5: 233–239. [DOI] [PubMed] [Google Scholar]
- Tseng JL, Baetge EE, Zurn AD, Aebischer P ( 1997) GDNF reduces drug-induced rotational behavior after medial forebrain bundle transection by a mechanism not involving striatal dopamine. J Neurosci 17: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S ( 2001) Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci USA 98: 2837–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK ( 2001) Caspase-9 activation results in down-stream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci 21: 9519–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KKW, Yuen P-W ( 1994) Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol 15: 412–419. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Conn PJ ( 2002) Dopamine modulates the function of group II and group III metabotropic glutamate receptors in the substantia nigra pars reticulata. J Pharmacol Exp Ther 302: 433–441. [DOI] [PubMed] [Google Scholar]
- Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB ( 2001) Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci USA 98: 10433–10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, Baimbridge KG, McGeer EG ( 1990) Relative sparing in Parkinson's disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res 526: 303–307. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Kohda Y, Tsuchiya K, Ueno T, Yamashita J, Yoshioka T, Kominami E ( 1998) Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on “calpain-cathepsin hypothesis”. Eur J Neurosci 10: 1723–1733. [DOI] [PubMed] [Google Scholar]
- Yang L, Matthews RT, Schulz JB, Klockgether T, Liao AW, Martinou JC, Penney Jr JB, Hyman BT, Beal MF ( 1998) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyride neurotoxicity is attenuated in mice overexpressing Bcl-2. J Neurosci 18: 8145–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman UJ, Boring L, Pak JH, Mukerjee N, Wang KK ( 2000) The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB Life 50: 63–68. [DOI] [PubMed] [Google Scholar]