Figure 2.
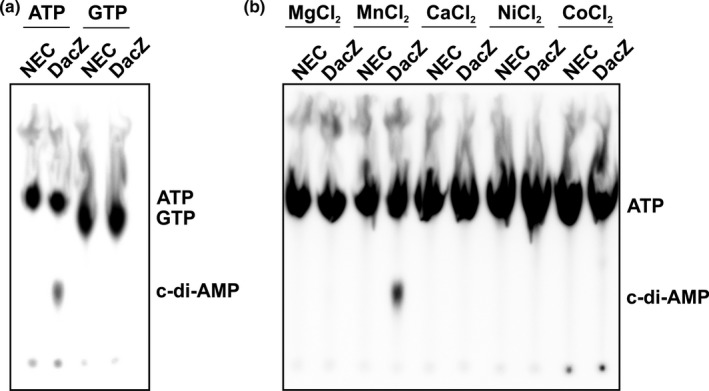
DAC activity measurements for substrate specify and metal cofactor usage of DacZ purified from Escherichia coli. (a) Phosphor screen of thin‐layer chromatography separating enzymatic products of in vitro DAC activity of DacZ using [α‐32P]ATP, respectively, [α‐32P]GTP as substrate. Figure represents one of three technical replicates with similar results. (b) Phosphor screen of thin‐layer chromatography separating enzymatic products of in vitro DAC activity of DacZ incubated with different metal‐(II) chloride solutions using [α‐32P]ATP as substrate. Figure represents one of three technical replicates with similar results. Position of cyclic di‐adenylate monophosphate (c‐di‐AMP) is indicated based on previous results from comparable assays (Bai et al., 2012) (NEC: non enzyme control)
