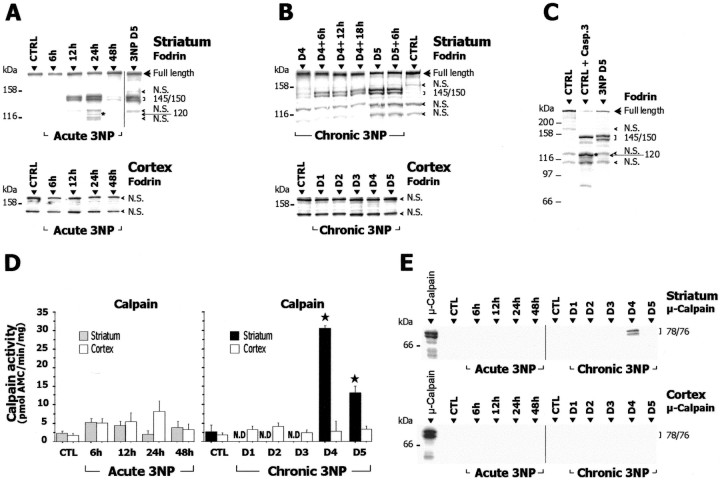
Figure 7.
Calpain activation in acute and chronic 3-NP models. A–C, Homogenates prepared from striatum and cerebral cortex of 3-NP-treated and control (CTRL) rats were analyzed by SDS-PAGE followed by Western blot for fodrin (known substrate of calpain and caspase-3) and assayed for calpain-dependent proteolytic activity using the fluorogenic substrate N-succinyl-LY-AMC (D). E, Western blot analysis ofμ-calpain cleavage in cytosol fractions from striatum and cerebral cortex of 3-NP-treated animals. The typical calpain-dependent cleavage of fodrin appearing as a 145/150 kDa doublet is found in the striatum of both acute (A) and chronic (B) 3-NP models (each lane contains pooled samples from 5–8 animals). Note in A the presence between two nonspecific (N.S.) bands of a 120 kDa band (asterisk), possibly because of a caspase-3-mediated fodrin breakdown product. In B, chronic 3-NP animals were analyzed every 6 hr showing that the intensity of this doublet increases on day 4 and is maximal at day 5. Western blots for fodrin in cerebral cortex were slightly overexposed. C shows that the pattern found in the striatum of 3-NP-treated animals (day 5) is clearly different from the caspase-3-mediated cleavage of fodrin observed after in vitro digestion of control samples with recombinant caspase-3 (CTRL + R. Casp-3). Note the prominent increase in the proteolytic activity of calpain and the presence of cleaved/active form ofμ-calpain in the striatum of chronic 3-NP rats, whereas no changes were found within the cerebral cortex of the same animals. Data are means ± SEM determined in 5–10 animals. N.D., Not detectable. ★p < 0.0005 compared with control, ANOVA, and post hoc Scheffé F test.