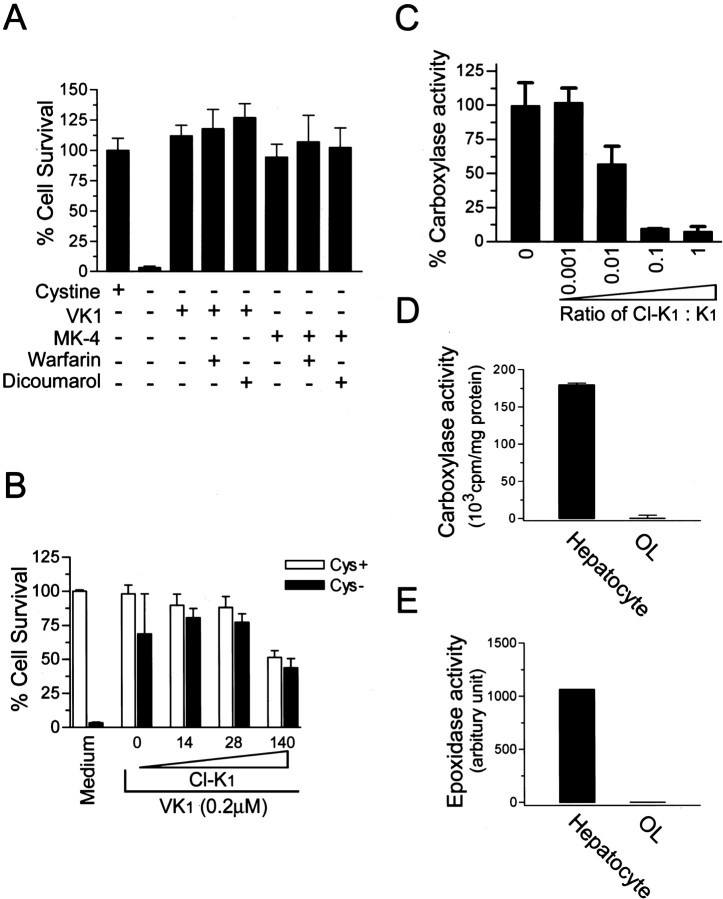
Figure 5.
Vitamin K protected OLs via a mechanism independent of vitamin K-dependentγ-glutamylcarboxylase.A,Inhibitors of vitamin K cycling, warfarin and dicoumarol, had no effect on vitamin K protection. OL precursors were induced to undergo cystine depletion-induced cell death in the presence or absence of indicated treatment for 24 hr, and cell viability was assayed. Concentrations used were: vitamin K1, 0.1μm; MK-4, 0.1μm; warfarin, 500μm; dicoumarol, 200μm. Results are representative of at least two separate experiments. B, Carboxylase inhibitor Cl-K1 did not reverse vitamin K protection. Cells were treated as indicated with or without Cl-K1 (0–140 μm) and vitamin K1 (0.2 μm) for 24 hr, and cell viability was analyzed. C, Cl-K1 directly inhibited enzymatic activity of purified γ-glutamylcarboxylase in vitro. Purified γ-glutamylcarboxylase was incubated in the presence of increasing concentrations of Cl-K1 versus K1 and assayed in duplicates for its enzyme activity. Results are mean ± SEM of three independent experiments. D, E, OL precursors express little, if any, γ-glutamylcarboxylase and vitamin K epoxidase activity. Microsomal proteins were extracted from cultured primary OLs, and vitamin K-dependent carboxylase and epoxidase activity of the microsomal preparation were analyzed. Primary rat hepatocyte microsomal preparation was performed in parallel as positive control for the enzyme assays. Results are representative of two independent experiments.