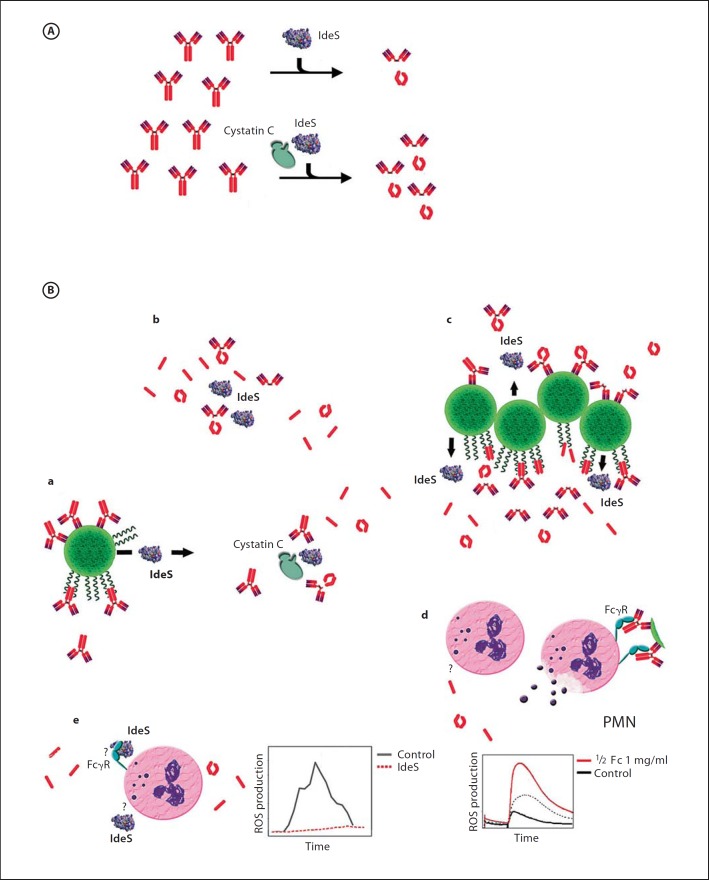
Fig. 1.
Overview of features and functions of streptococcal IdeS. A Powering IdeS activity. Interaction of IdeS with the abundant cysteine protease inhibitor cystatin C accelerates IgG endopeptidase activity. B IdeS biological activity. a S. pyogenes, recognized by specific IgG and/or binding IgG to M protein at the streptococcal surface, secrete active IdeS. b In the circulation, IdeS cleaves IgG in the hinge region, generating single-cleaved inactive IgG, F(ab′)2 fragments and two identical ½Fc fragments. c Specific IgG at the streptococcal surface is efficiently cleaved by IdeS, leaving antigen bound F(ab′)2 fragments that protect streptococcal surface antigens and releasing ½Fc fragments. Fc-bound IgG is also cleaved, releasing F(ab′)2 fragments and, due to their low affinity for M-protein, ½Fc fragments into the circulation. Free binding sites at M protein can bind new IgG. d Circulating Fc fragments prime polymorphonuclear leukocytes (PMN) to an increased and fast production of ROS by binding to a yet-unknown receptor. Upon activation by immunocomplexes, these PMNs discharge and degranulate at a distance from the infection site without harming the bacteria. e Suppression of ROS production by IdeS to prevent PMNs to discharge close to the bacteria; IdeS suppresses ROS production, independently from its IgG endopeptidase activity. The precise mechanism so far remains elusive, but might be mediated by interaction with FcγR.