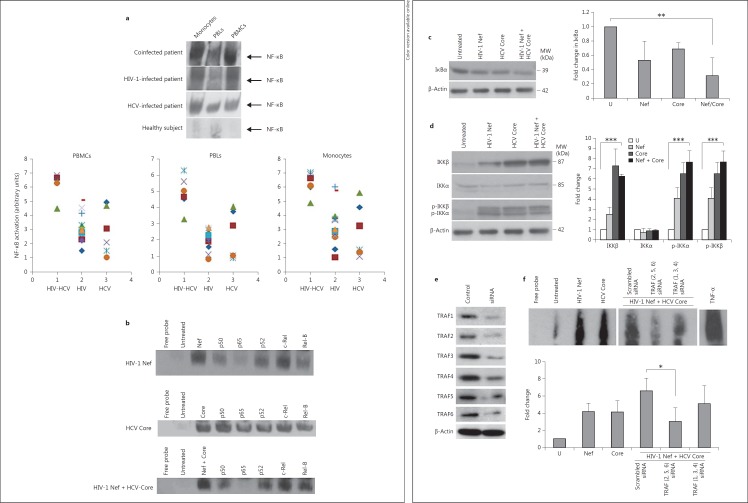
Fig. 6.
Enhanced NF-κB activation in MDMs treated with HIV-1 Nef and HCV Core is mediated through TRAF2, TRAF5 and TRAF6. a Highest NF-κB activation in monocytes and PBMCs isolated from HIV-HCV-coinfected subjects. Upper panel: data are representative of NF-κB activation determined by EMSA in PBMCs, monocytes and PBLs isolated from the peripheral blood of coinfected patients (n = 6), HIV-infected patients (n = 13), HCV-infected patients (n = 6) and normal healthy donors (n = 3). Lower panel: NF-κB activation measured by EMSA and quantified by densitometry using ImageJ 1.40 software in PBMCs, monocytes and PBLs isolated from the peripheral blood of HIV-HCV-coinfected patients (n = 6), HIV-infected patients (n = 13), and HCV-infected patients (n = 6). The level of NF-κB in cells of healthy donors was arbitrarily established at 1. b Enhanced NF-κB activation in MDMs treated with HIV-1 rNef, HCV rCore and HIV-1 rNef + HCV rCore. MDM cultures were treated with HIV-1 rNef (100 ng/ml), HCV rCore protein (100 ng/ml) or HIV-1 rNef (100 ng/ml) + HCV rCore protein (100 ng/ml) and nuclear extracts were prepared at 30 min posttreatment. NF-κB activation was determined by EMSA. Interference of NF-κB activation in response to HIV-1 rNef and HCV rCore by Abs against NF-κB subunits: nuclear extracts from treated MDMs were incubated for 20 min with anti-p50, anti-p65, anti-p52, anti-c-Rel and anti-RelB Abs, and then assayed for NF-κB binding activity by EMSA. Results are representative of two independent experiments. c IkBα degradation in MDMs treated with HIV-1 rNef and HCV rCore. Cytoplasmic extracts were prepared from MDMs treated with HIV-1 rNef (100 ng/ml), HCV rCore (100 ng/ml) or HIV-1 rNef (100 ng/ml) + HCV rCore protein (100 ng/ml) for 30 min and then assayed for the expression of IkBα by Western blot. β-actin was used as a loading control. Protein levels were quantified by densitometry using ImageJ 1.40 software (the level of protein in mock cells was arbitrarily established at 1). Results represent means ± SD of threeindependent experiments. ** p < 0.01. d IKKα and IKKβ expression and phosphorylation in MDMs treated with HIV-1 rNef and HCV rCore. Cytoplasmic extracts were prepared from MDMs treated with HIV-1 rNef (100 ng/ml), HCV rCore (100 ng/ml) or HIV-1 rNef (100 ng/ml) + HCV rCore protein (100 ng/ml) for 30 min and then assayed for the corresponding protein by Western blot. β-actin was used as a loading control. Protein levels were quantified by densitometry using ImageJ 1.40 software (the level of protein in mock cells was arbitrarily established at 1). Results represent means ± SD of threeindependent experiments. *** p < 0.001. e Knockdown of TRAF1-TRAF6 proteins by siRNA in MDMs. MDM cultures were transfected with TRAF1-TRAF6 siRNAs or scrambled control. Total cellular extracts were prepared 48 h posttransfection. Protein expression was analyzed by Western blot. β-actin was used as a loading control. f NF-κB activation in MDMs treated with HIV-1 rNef and HCV rCore depends on TRAF2, TRAF5 and TRAF6. 48 h before treatment with HIV-1 rNef (100 ng/ml) and HCV rCore (100 ng/ml), MDMs were transfected with a mixture of TRAF2/TRAF5/TRAF6 siRNAs, a mixture of TRAF1/TRAF3/TRAF4 siRNAs, or a scrambled control. NF-κB activation was determined by EMSA. Nuclear extracts of MDMs treated with TNFα (10 ng/ml) were used as a positive control. The histogram represents the quantification of NF-κB activity by densitometry using ImageJ 1.40 software (the level of NF-κB activity in mock cells was arbitrarily established at 1). Results represent means ± SD of three independent experiments. * p < 0.05.