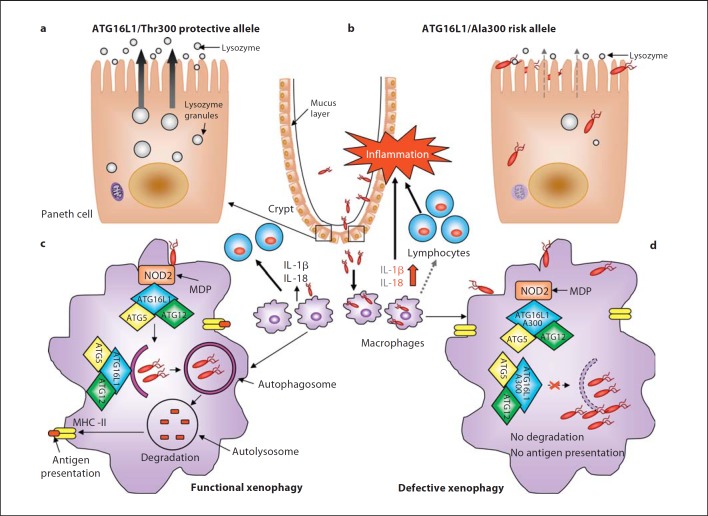
Fig. 1.
Impact of the CD-associated ATG16L1/Ala300 variant on xenophagy-mediated intracellular bacteria clearance during CD onset. a, b Paneth cells with the ATG16L1/Ala300 risk allele exhibit abnormalities in structure and function compared to Paneth cells with the ATG16L1/Thr300 protective allele such as decreased and disorganized granule compartments with disrupted granule exocytosis pathway (grey dotted arrow) and degraded mitochondria, leading to reduced lysozymes in the mucus layer. This could probably confer the intestinal epithelial layer susceptibility to microbial infection, leading to bacterial overgrowth and the invasion of commensal bacteria, and predominantly of pathogenic bacteria with invasive properties. c Invading bacteria are detected by macrophages and dendritic cells. NOD2 in macrophages senses MDP and recruits ATG16L1 to the plasma membrane at the bacterial entry site, initiating functional xenophagy with the recruitment of the ATG16-ATG5-ATG12 complex to the membrane of autophagosomes. Bacteria are degraded inside autophagolysosomes and the produced peptides are loaded to the MHC class II for antigen presentation, which will be recognized by CD4+ T cells. Wild-type macrophages and dendritic cells detecting invasive bacteria also secrete inflammatory cytokines IL-1β and IL-18. d In macrophages and dendritic cells with the ATG16L1/Ala300 risk alleles, the mutated ATG16L1 protein is still able to bind to NOD2 upon MDP stimulation and able to recruit the ATG12-ATG5 complex, but fails to induce xenophagy. Autophagy-deficient macrophages fail to kill intracellular bacteria and to present antigen by MHC class II, leading to inappropriate activation of the adaptive immune system (grey dotted arrow). Autophagy-deficient macrophages produce high levels of IL-1β and IL-18 (red arrow). These result in severe inflammation and consequently chronic inflammatory status, which occur during CD development.